Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Electrical Conductivity on the Electrochemical Performances of Layered Structure Lithium Trivanadate (LiV3–xMxO8, M= Zn/Co/Fe/Sn/Ti/Zr/Nb/Mo, x = 0.01–0.1) as Cathode Materials for Energy Storage
ACS Omega ( IF 3.7 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acsomega.7b01904 P. Senthil Kumar 1, 2, 3 , Sakunthala Ayyasamy 2 , Eng Soon Tok 1 , Stefan Adams 4 , M. V. Reddy 1, 3, 4
ACS Omega ( IF 3.7 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acsomega.7b01904 P. Senthil Kumar 1, 2, 3 , Sakunthala Ayyasamy 2 , Eng Soon Tok 1 , Stefan Adams 4 , M. V. Reddy 1, 3, 4
Affiliation
|
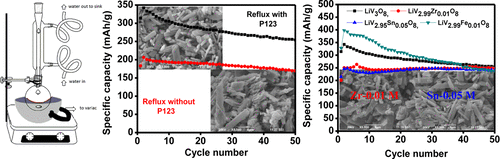
Pristine trivanadate (LiV3O8) and doped lithium trivanadate (LiV3–xMxO8, M = Zn/Co/Fe/Sn/Ti/Zr/Nb/Mo, x = 0.01/0.05/0.1 M) compounds were prepared by a simple reflux method in the presence of the polymer, Pluronic P123, as the chelating agent. For comparison, pristine LiV3O8 alone was also prepared in the absence of the chelating agent. The Rietveld-refined X-ray diffraction patterns shows all compounds to exist in the layered monoclinic LiV3O8 phase belonging to the space group of P21/m. Scanning electron microscopy analysis shows the particles to exhibit layers of submicron-sized particles. The electrochemical performances of the coin cells were compared at a current density of 30 mA/g in the voltage window of 2–4 V. The cells made with compounds LiV2.99Zr0.01O8 and LiV2.95Sn0.05O8 show a high discharge capacity of 245 ± 5 mA h/g, with an excellent stability of 98% at the end of the 50th cycle. The second cycle discharge capacity of 398 mA h/g was obtained for the compound LiV2.99Fe0.01O8, and its capacity retention was found to be 58% after 50 cycles. The electrochemical performances of the cells were correlated with the electrical properties and the changes in the structural parameters of the compounds.
中文翻译:

电导率对层状结构三钒酸锂(LiV 3– x M x O 8,M = Zn / Co / Fe / Sn / Ti / Zr / Nb / Mo,x = 0.01-0.1)的电化学性能的影响用于储能
原始三钒酸盐(LiV 3 O 8)和掺杂的三钒酸锂(LiV 3– x M x O 8,M = Zn / Co / Fe / Sn / Ti / Zr / Nb / Mo,x = 0.01 / 0.05 / 0.1 M)在聚合物Pluronic P123作为螯合剂的存在下,通过简单的回流方法制备了氯丁酮。为了比较,在不存在螯合剂的情况下也单独制备了原始的LiV 3 O 8。Rietveld精制的X射线衍射图表明,所有化合物均存在于层状单斜LiV 3 O 8相中,该相属于P 2 1 / m的空间群。扫描电子显微镜分析显示颗粒显示出亚微米级颗粒层。在2–4 V的电压窗口中以30 mA / g的电流密度比较了币形电池的电化学性能。用化合物LiV 2.99 Zr 0.01 O 8和LiV 2.95 Sn 0.05 O 8制成的电池显示出高放电容量为245±5 mA h / g,在第50个周期结束时具有98%的出色稳定性。化合物LiV 2.99 Fe 0.01 O 8的第二循环放电容量为398 mA h / g,在50个循环后发现其容量保留率为58%。电池的电化学性能与化合物的电学性质和结构参数的变化相关。
更新日期:2018-03-13
中文翻译:

电导率对层状结构三钒酸锂(LiV 3– x M x O 8,M = Zn / Co / Fe / Sn / Ti / Zr / Nb / Mo,x = 0.01-0.1)的电化学性能的影响用于储能
原始三钒酸盐(LiV 3 O 8)和掺杂的三钒酸锂(LiV 3– x M x O 8,M = Zn / Co / Fe / Sn / Ti / Zr / Nb / Mo,x = 0.01 / 0.05 / 0.1 M)在聚合物Pluronic P123作为螯合剂的存在下,通过简单的回流方法制备了氯丁酮。为了比较,在不存在螯合剂的情况下也单独制备了原始的LiV 3 O 8。Rietveld精制的X射线衍射图表明,所有化合物均存在于层状单斜LiV 3 O 8相中,该相属于P 2 1 / m的空间群。扫描电子显微镜分析显示颗粒显示出亚微米级颗粒层。在2–4 V的电压窗口中以30 mA / g的电流密度比较了币形电池的电化学性能。用化合物LiV 2.99 Zr 0.01 O 8和LiV 2.95 Sn 0.05 O 8制成的电池显示出高放电容量为245±5 mA h / g,在第50个周期结束时具有98%的出色稳定性。化合物LiV 2.99 Fe 0.01 O 8的第二循环放电容量为398 mA h / g,在50个循环后发现其容量保留率为58%。电池的电化学性能与化合物的电学性质和结构参数的变化相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号