当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-Lactoglobulin Peptide Fragments Conjugated with Caffeic Acid Displaying Dual Activities for Tyrosinase Inhibition and Antioxidant Effect
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00050 Jin-Kyoung Yang 1 , Eunjin Lee 2 , In-Jun Hwang 1 , DaBin Yim 1 , Juhee Han 1 , Yoon-Sik Lee 2 , Jong-Ho Kim 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00050 Jin-Kyoung Yang 1 , Eunjin Lee 2 , In-Jun Hwang 1 , DaBin Yim 1 , Juhee Han 1 , Yoon-Sik Lee 2 , Jong-Ho Kim 1
Affiliation
|
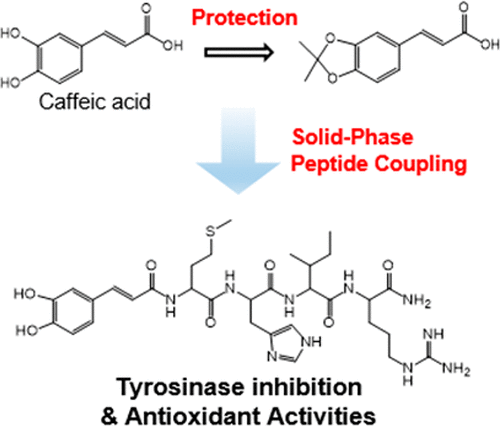
The regulation of tyrosinase activity and reactive oxygen species is of great importance for the prevention of dermatological disorders in the fields of medicine and cosmetics. Herein, we report a strategy based on solid-phase peptide chemistry for the synthesis of β-lactoglobulin peptide fragment/caffeic acid (CA) conjugates (CA-Peps) with dual activities of tyrosinase inhibition and antioxidation. The purity of the prepared conjugates, CA-MHIR, CA-HIRL, and CA-HIR, significantly increased to 99%, as acetonide-protected CA was employed in solid-phase coupling reactions on Rink amide resins. The tyrosinase inhibitory activities of all CA-Pep derivatives were higher than the activity of kojic acid, and CA-MHIR exhibited the highest tyrosinase inhibition activity (IC50 = 47.9 μM). Moreover, CA-Pep derivatives displayed significantly enhanced antioxidant activities in the peroxidation of linoleic acid as compared to the pristine peptide fragments. All CA-Pep derivatives showed no cytotoxicity against B16–F1 melanoma cells.
中文翻译:

与咖啡酸结合的β-乳球蛋白肽片段具有酪氨酸酶抑制和抗氧化作用的双重活性
酪氨酸酶活性和活性氧的调节对于预防医学和化妆品领域的皮肤病非常重要。本文中,我们报告了一种基于固相肽化学的策略,用于合成具有酪氨酸酶抑制和抗氧化双重活性的β-乳球蛋白肽片段/咖啡酸(CA)共轭物(CA-Peps)。制备的共轭物CA-MHIR,CA-HIRL和CA-HIR的纯度显着提高到99%,这是因为在Rink酰胺树脂上的固相偶合反应中使用了丙酮化物保护的CA。所有CA-Pep衍生物的酪氨酸酶抑制活性均高于曲酸的活性,并且CA-MHIR表现出最高的酪氨酸酶抑制活性(IC 50= 47.9μM)。此外,与原始肽片段相比,CA-Pep衍生物在亚油酸的过氧化中显示出显着增强的抗氧化活性。所有CA-Pep衍生物对B16–F1黑色素瘤细胞均无细胞毒性。
更新日期:2018-03-13
中文翻译:

与咖啡酸结合的β-乳球蛋白肽片段具有酪氨酸酶抑制和抗氧化作用的双重活性
酪氨酸酶活性和活性氧的调节对于预防医学和化妆品领域的皮肤病非常重要。本文中,我们报告了一种基于固相肽化学的策略,用于合成具有酪氨酸酶抑制和抗氧化双重活性的β-乳球蛋白肽片段/咖啡酸(CA)共轭物(CA-Peps)。制备的共轭物CA-MHIR,CA-HIRL和CA-HIR的纯度显着提高到99%,这是因为在Rink酰胺树脂上的固相偶合反应中使用了丙酮化物保护的CA。所有CA-Pep衍生物的酪氨酸酶抑制活性均高于曲酸的活性,并且CA-MHIR表现出最高的酪氨酸酶抑制活性(IC 50= 47.9μM)。此外,与原始肽片段相比,CA-Pep衍生物在亚油酸的过氧化中显示出显着增强的抗氧化活性。所有CA-Pep衍生物对B16–F1黑色素瘤细胞均无细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号