Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-03-14 , DOI: 10.1016/j.actbio.2018.03.012 Tao Li , Mingzheng Peng , Zezheng Yang , Xiaojun Zhou , Yuan Deng , Chuan Jiang , Ming Xiao , Jinwu Wang
|
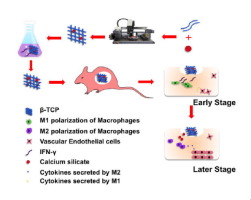
To promote vascularization of tissue-engineered bone, IFN-γ polarizing macrophages to M1 was loaded on 5% calcium silicate/β-tricalcium phosphate (CaSiO3-β-TCP) scaffolds. IFN-γ and Si released from the scaffold were designed to polarize M1 and M2 macrophages, respectively. β-TCP, CaSiO3-β-TCP, and IFN-γ@CaSiO3-β-TCP were fabricated and biocompatibilities were evaluated. Polarizations of macrophages were detected by flow cytometry. Human umbilical vein endothelial cells with GFP were cultured and induced on Matrigel with conditioned culture medium extracted from culture of macrophages loaded on scaffolds for evaluating angiogenesis. Four weeks after the scaffolds were subcutaneously implanted into C57B1/6, vascularization was evaluated by visual observation, hematoxylin and eosin staining, as well as immunohistochemistry of CD31. The results showed that IFN-γ@CaSiO3-β-TCP scaffolds released IFN-γ in the early stage (1–3 days) to stimulate macrophages to M1 polarization, followed by release of Si inducing macrophages to M2 polarization while scaffolds degraded. The activation of M1/M2 allows macrophages to secrete more cytokines, including VEGF, CXCL12 and PDGF-BB. The IFN-γ@CaSiO3-β-TCP scaffolds formed more blood vessels in vitro and in vivo compared to the control groups. The study indicated that the design of tissue-engineered scaffolds with immunomodulatory function utilized host macrophages to increase vascularization of tissue-engineered bone, providing a new strategy for accelerating vascularization and osteogenesis of tissue-engineered scaffolds and showing the potential for treatment of major bone defects.
Statement of significance
A 3-D printed immunomodulatory scaffold was designed for repair of massive bone defects. Through the release of interferon γ and silicon ions, the new immunomodulatory scaffold promoted the M1 and M2 polarization of macrophages, boosting angiogenesis. This scaffold provided a new strategy for accelerating vascularization and osteogenesis of tissue-engineered scaffolds and showing the potential for treatment of major bone defects.
中文翻译:

3D打印的加载IFN-γ的硅酸钙-β-磷酸三钙支架依次激活巨噬细胞的M1和M2极化,从而促进组织工程骨的血管形成
为了促进组织工程骨骼的血管生成,将IFN-γ极化巨噬细胞(至M1)加载到5%硅酸钙/β-磷酸三钙(CaSiO 3 - β -TCP)支架上。从支架释放的IFN-γ和Si分别用于极化M1和M2巨噬细胞。β-TCP,CaSiO 3 - β-TCP和IFN-γ@ CaSiO 3制备了-β-TCP并评估了生物相容性。通过流式细胞仪检测巨噬细胞的极化。培养具有GFP的人脐静脉内皮细胞,并在Matrigel上诱导,用条件培养基从提取自负载在支架上的巨噬细胞培养物中提取,以评估血管生成。将支架皮下植入C57B1 / 6后四周,通过肉眼观察,苏木精和曙红染色以及CD31的免疫组织化学评估血管形成。结果表明,IFN-γ@ CaSiO 3-β-TCP支架在早期(1-3天)释放IFN-γ刺激巨噬细胞至M1极化,随后释放Si诱导巨噬细胞向M2极化,而支架降解。M1 / M2的激活使巨噬细胞分泌更多的细胞因子,包括VEGF,CXCL12和PDGF-BB。的IFN-γ@卡西欧3 -β-TCP支架材料形成更多的血管的体外和体内相比于对照组。研究表明,具有免疫调节功能的组织工程支架的设计利用宿主巨噬细胞来增加组织工程骨的血管生成,为加速组织工程支架的血管生成和成骨提供了新的策略,并显示了治疗主要骨缺损的潜力。
重要声明
设计了3-D打印的免疫调节支架,用于修复大量的骨缺损。通过释放干扰素γ和硅离子,新的免疫调节支架促进了巨噬细胞的M1和M2极化,促进了血管生成。该支架为加速组织工程支架的血管形成和成骨提供了新的策略,并显示了治疗主要骨缺损的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号