Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-03-13 , DOI: 10.1016/j.bmcl.2018.03.031 De-Kun Hu , Dong-Sheng Zhao , Min He , Hong-Wei Jin , Yong-Mei Tang , Lian-Hui Zhang , Gao-Peng Song , Zi-Ning Cui
|
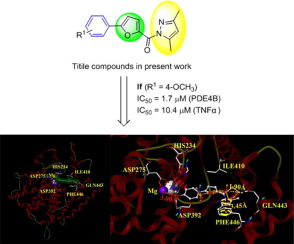
A series of 3,5-dimethylpyrazole derivatives containing 5-phenyl-2-furan moiety were designed and synthesized as phosphodiesterase type 4 (PDE4) inhibitors. Bioassay results showed that the title compounds exhibited considerable inhibitory activity against PDE4B and blockade of LPS-induced TNFα release. Among the designed compounds, compound If showed the best inhibitory activity against PDE4B with the IC50 value of 1.7 μM, which also showed good in vivo activity in animal models of asthma/COPD and sepsis induced by LPS. The primary structure–activity relationship (SAR) study and docking results suggested that introduction of the substituent groups to the phenyl ring at the para-position, especially methoxy group, was helpful to enhance inhibitory activity against PDE4B.
中文翻译:

3,5-二甲基吡唑衍生物作为潜在的PDE4抑制剂的合成及生物活性
设计并合成了一系列含有5-苯基-2-呋喃部分的3,5-二甲基吡唑衍生物,作为4型磷酸二酯酶(PDE4)抑制剂。生物测定结果表明,该标题化合物显示出相当大的抑制活性对PDE4B和LPS诱导的TNF阻断α释放。在设计的化合物中,化合物If对PDE4B表现出最佳的抑制活性,IC 50值为1.7μM,在哮喘/ COPD和LPS诱发的败血症动物模型中也显示出良好的体内活性。初步的结构-活性关系(SAR)研究和对接结果表明,在对位苯环上引入取代基位置,特别是甲氧基,有助于增强对PDE4B的抑制活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号