当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mutual Solubilities of Cyclohexane and Water in Aqueous Methyldiethanolamine /Cyclohexane Liquid–Liquid Equilibria
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.jced.7b00873 Rana Danon 1 , Maaike C. Kroon 1 , Fawzi Banat 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.jced.7b00873 Rana Danon 1 , Maaike C. Kroon 1 , Fawzi Banat 1
Affiliation
|
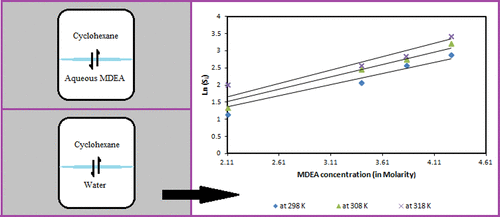
The aim of this work is to study the solubility of cyclohexane in aqueous methyldiethanolamine (MDEA) solutions. Thermostated jacketed double glass equilibrium cells were used to measure the mutual solubility between two liquid phases of aqueous MDEA and cyclohexane at 298–318 K under atmospheric pressure. The cyclohexane solubility in the aqueous liquid samples was analyzed using GC–MS coupled with purge and trap technique, while that of water in the cyclohexane-rich phase was measured via the Karl-Fischer titration method. It was observed that the solubility of cyclohexane in the aqueous phase increases with increasing temperature. Furthermore, an increase in amine composition, increases the solubility of cyclohexane. The mutual water solubility in the cyclohexane-rich phase increases rapidly with increasing temperature while it was rather nonsensitive toward the amine composition. A simple correlation for the salting-in ratio has been used that allows for a direct comparison between the solubilities of cyclohexane in aqueous solutions of MDEA to those in pure water at various temperatures.
中文翻译:

甲基二乙醇胺/环己烷液-液平衡中环己烷和水的互溶性
这项工作的目的是研究环己烷在甲基二乙醇胺(MDEA)水溶液中的溶解度。恒温夹套双层玻璃平衡池用于在大气压下在298–318 K下测量MDEA水溶液和环己烷的两个液相之间的互溶性。使用GC-MS结合吹扫和捕集技术分析了含水液体样品中的环己烷溶解度,而通过Karl-Fischer滴定法测量了富含环己烷的水的溶解度。观察到环己烷在水相中的溶解度随温度升高而增加。此外,胺组成的增加增加了环己烷的溶解度。富环己烷相中的互溶性随温度的升高而迅速增加,而对胺成分则不敏感。已使用简单的盐化比例相关性,可以直接比较在不同温度下MDEA水溶液中环己烷与纯水中的环己烷溶解度。
更新日期:2018-03-14
中文翻译:

甲基二乙醇胺/环己烷液-液平衡中环己烷和水的互溶性
这项工作的目的是研究环己烷在甲基二乙醇胺(MDEA)水溶液中的溶解度。恒温夹套双层玻璃平衡池用于在大气压下在298–318 K下测量MDEA水溶液和环己烷的两个液相之间的互溶性。使用GC-MS结合吹扫和捕集技术分析了含水液体样品中的环己烷溶解度,而通过Karl-Fischer滴定法测量了富含环己烷的水的溶解度。观察到环己烷在水相中的溶解度随温度升高而增加。此外,胺组成的增加增加了环己烷的溶解度。富环己烷相中的互溶性随温度的升高而迅速增加,而对胺成分则不敏感。已使用简单的盐化比例相关性,可以直接比较在不同温度下MDEA水溶液中环己烷与纯水中的环己烷溶解度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号