当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Domino Reaction of Pyrrolidinium Ylides: Michael Addition/[1,2]-Stevens Rearrangement
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.joc.7b03278 Anna Kowalkowska 1 , Andrzej Jończyk 1 , Jan K. Maurin 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.joc.7b03278 Anna Kowalkowska 1 , Andrzej Jończyk 1 , Jan K. Maurin 2, 3
Affiliation
|
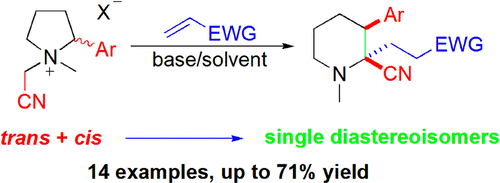
A novel domino reaction featuring a Michael addition/[1,2]-Stevens rearrangement reaction of pyrrolidinium ylides with electrophilic alkenes is described. Ylides generated under mild conditions from 2-aryl-N-cyanomethyl-N-methylpyrrolidinium salts entered the Michael addition, followed by a [1,3]-hydrogen shift and finally the [1,2]-Stevens rearrangement to give 3-aryl-2-cyano-2-(2-EWG-ethyl)-1-methylpiperidines.
中文翻译:

吡咯烷鎓叶立德的多米诺反应:迈克尔加成/ [1,2]-史蒂文斯重排
描述了一种新颖的多米诺骨牌反应,其特征在于吡咯烷鎓烷基化物与亲电子烯烃的迈克尔加成/ [1,2]-史蒂文斯重排反应。在温和条件下由2-芳基-N-氰甲基-N-甲基吡咯烷鎓盐生成的叶立德进入Michael加成反应,随后发生[1,3]-氢转移,最后发生[1,2] -Stevens重排,得到3-芳基-2-氰基-2-(2-EWG-乙基)-1-甲基哌啶。
更新日期:2018-03-13
中文翻译:

吡咯烷鎓叶立德的多米诺反应:迈克尔加成/ [1,2]-史蒂文斯重排
描述了一种新颖的多米诺骨牌反应,其特征在于吡咯烷鎓烷基化物与亲电子烯烃的迈克尔加成/ [1,2]-史蒂文斯重排反应。在温和条件下由2-芳基-N-氰甲基-N-甲基吡咯烷鎓盐生成的叶立德进入Michael加成反应,随后发生[1,3]-氢转移,最后发生[1,2] -Stevens重排,得到3-芳基-2-氰基-2-(2-EWG-乙基)-1-甲基哌啶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号