当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Aerobic Oxidative Coupling of Allylic Alcohols with Anilines in the Synthesis of Nitrogen Heterocycles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.joc.8b00287 Gangam Srikanth Kumar 1 , Diksha Singh 1 , Manish Kumar 1 , Manmohan Kapur 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.joc.8b00287 Gangam Srikanth Kumar 1 , Diksha Singh 1 , Manish Kumar 1 , Manmohan Kapur 1
Affiliation
|
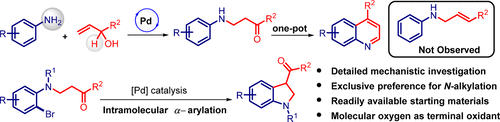
We report herein an unprecedented and expedient Pd-catalyzed oxidative coupling of allyl alcohols with anilines to afford β-amino ketones which are converted into substituted quinolines in a one-pot fashion. The exclusive preference for N-alkylation over N-allylation makes this approach unique when compared to those reported in literature. Detailed mechanistic investigations reveal that the conjugate addition pathway was the predominant one over the allylic amination pathway. The notable aspects of the present approach are the use of readily available, bench-stable allyl alcohols and molecular oxygen as the terminal oxidant, in the process dispensing the need for unstable and costly enones. Further, we explored the synthetic utility of β-amino ketones through an intramolecular α-arylation methodology and a one-pot domino annulation, thereby providing rapid access to indolines and quinolines.
中文翻译:

钯催化的氮杂杂环合成中烯丙醇与苯胺的好氧氧化偶联
我们在本文中报道了烯丙醇与苯胺的空前和方便的Pd催化氧化偶联,以提供β-氨基酮,将其以一锅法转化为取代的喹啉。为独家偏好ň烷基化过ñ与文献报道的方法相比,烯丙基化使这种方法具有独特性。详细的机械研究表明,共轭添加途径是烯丙基胺化途径中最主要的途径。本方法的显着方面是在该方法中使用容易获得的,稳定的烯丙醇和分子氧作为末端氧化剂,从而消除了对不稳定和昂贵的烯酮的需求。此外,我们通过分子内α-芳基化方法和一锅多米诺法研究了β-氨基酮的合成效用,从而提供了快速获得二氢吲哚和喹啉的途径。
更新日期:2018-03-13
中文翻译:

钯催化的氮杂杂环合成中烯丙醇与苯胺的好氧氧化偶联
我们在本文中报道了烯丙醇与苯胺的空前和方便的Pd催化氧化偶联,以提供β-氨基酮,将其以一锅法转化为取代的喹啉。为独家偏好ň烷基化过ñ与文献报道的方法相比,烯丙基化使这种方法具有独特性。详细的机械研究表明,共轭添加途径是烯丙基胺化途径中最主要的途径。本方法的显着方面是在该方法中使用容易获得的,稳定的烯丙醇和分子氧作为末端氧化剂,从而消除了对不稳定和昂贵的烯酮的需求。此外,我们通过分子内α-芳基化方法和一锅多米诺法研究了β-氨基酮的合成效用,从而提供了快速获得二氢吲哚和喹啉的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号