当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Regioselective C–H Functionalization of Arenes Substituted by Two N-Heterocycles and Application in Late-Stage Functionalization
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.joc.8b00322 Da-Wei Yin 1 , Gang Liu 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.joc.8b00322 Da-Wei Yin 1 , Gang Liu 1, 2
Affiliation
|
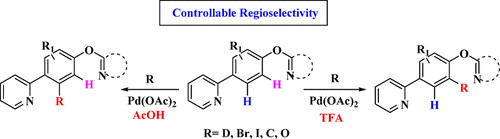
Reported herein is a Pd-catalyzed regioselective C–H activation method that is used for C–H deuteration, carbonylation, halogenation, and oxidation of arene substrates substituted by two N-heterocycles. When conducted in acetic acid (AcOH), these reactions occur at the five-membered palladacycle sites, whereas they switch to the six-membered palladacycle sites in trifluoroacetic acid (TFA). This controllable regioselective C–H activation is applied for late-stage functionalization of bioactive molecules. A mechanism study indicated that the regioselectivity is achieved by Brönsted acid–Lewis base interactions and electronic effects (in TFA) and the different kinetic stabilities of palladacycle intermediates (in AcOH).
中文翻译:

钯催化的两个N-杂环取代的芳烃的区域选择性C–H功能化及其在后期功能化中的应用
本文报道了一种钯催化的区域选择性C–H活化方法,用于C–H氘代,羰基化,卤化和氧化被两个N-杂环取代的芳烃底物。当在乙酸(AcOH)中进行时,这些反应发生在五元的Palladacycle位置,而它们切换到三氟乙酸(TFA)中的六元的Palladacycle位置。这种可控的区域选择性C–H激活被用于生物活性分子的后期功能化。一项机理研究表明,区域选择性是通过布朗斯台德酸-刘易斯碱相互作用和电子效应(在TFA中)以及palladacycle中间体的不同动力学稳定性(在AcOH中)实现的。
更新日期:2018-03-13
中文翻译:

钯催化的两个N-杂环取代的芳烃的区域选择性C–H功能化及其在后期功能化中的应用
本文报道了一种钯催化的区域选择性C–H活化方法,用于C–H氘代,羰基化,卤化和氧化被两个N-杂环取代的芳烃底物。当在乙酸(AcOH)中进行时,这些反应发生在五元的Palladacycle位置,而它们切换到三氟乙酸(TFA)中的六元的Palladacycle位置。这种可控的区域选择性C–H激活被用于生物活性分子的后期功能化。一项机理研究表明,区域选择性是通过布朗斯台德酸-刘易斯碱相互作用和电子效应(在TFA中)以及palladacycle中间体的不同动力学稳定性(在AcOH中)实现的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号