当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From Foldable Open-Chains to Shape-Persistent Macrocycles: Synthesis, Impact on 2D Ordering, and Stimulated Self-Assembly
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-13 , DOI: 10.1021/jacs.8b01805 Soobin Kim 1 , Henry D. Castillo 2 , Milim Lee 1 , Riley D. Mortensen 2 , Steven L. Tait 2 , Dongwhan Lee 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-13 , DOI: 10.1021/jacs.8b01805 Soobin Kim 1 , Henry D. Castillo 2 , Milim Lee 1 , Riley D. Mortensen 2 , Steven L. Tait 2 , Dongwhan Lee 1
Affiliation
|
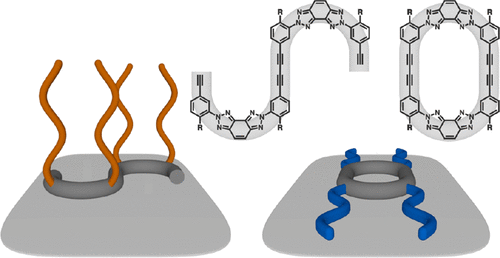
Small molecule self-assembly at surfaces offers an efficient route to highly ordered organic films that can be programmed for a variety of chemical and electronic applications. The success of these materials depends on the ability to program intermolecular interactions to guide precise structural ordering. Toward this objective, we have designed and synthesized a series of bis(triazolo)benzene-based π-conjugated molecules. Our synthesis exploits a last-stage C-C cross-coupling reaction to close up zigzag-shaped linear precursors to cyclized products, so that direct side-by-side comparisons can be made for their structure-dependent self-assembly behavior at surfaces and response to external stimuli. Indeed, scanning tunneling microscopy (STM) analysis revealed distinct differences as the conformational flexibility of the molecular backbone and the chemical structure of the peripheral groups are varied. Specifically, alkyl chains adsorb and form interdigitated structures, whereas oligo ethylene glycol (OEG) chains remain desorbed and thus shift self-assembly to more densely packed π-conjugated cores. While the macrocycles self-assemble immediately and spontaneously, their linear precursors exhibit slower self-assembly kinetics, which could be attributed to the difference in the degree of conformational freedom. We also found that perturbation by the STM tip and the addition of cosolutes profoundly impacted the kinetics of self-assembly and surface patterning. This highly unusual behavior highlights the importance of noncovalent interactions that are inherently weak in solution but can be made strong for symmetric and conformationally restricted molecules confined within 2D surfaces.
中文翻译:

从可折叠的开链到形状持久的大环:合成、对二维排序的影响和受激自组装
表面的小分子自组装为高度有序的有机薄膜提供了一种有效的途径,可以为各种化学和电子应用编程。这些材料的成功取决于对分子间相互作用进行编程以指导精确结构排序的能力。为实现这一目标,我们设计并合成了一系列基于双(三唑)苯的 π 共轭分子。我们的合成利用最后阶段的 CC 交叉偶联反应将锯齿形线性前体封闭为环化产物,因此可以直接并排比较它们在表面的结构依赖性自组装行为和响应外部刺激。确实,扫描隧道显微镜 (STM) 分析显示出明显的差异,因为分子主链的构象灵活性和外围基团的化学结构各不相同。具体而言,烷基链吸附并形成叉指结构,而低聚乙二醇 (OEG) 链保持解吸状态,从而将自组装转移到更密集的 π 共轭核。虽然大环能立即自组装,但它们的线性前体表现出较慢的自组装动力学,这可能归因于构象自由度的差异。我们还发现 STM 尖端的扰动和 cosolutes 的添加深刻影响了自组装和表面图案化的动力学。
更新日期:2018-03-13
中文翻译:

从可折叠的开链到形状持久的大环:合成、对二维排序的影响和受激自组装
表面的小分子自组装为高度有序的有机薄膜提供了一种有效的途径,可以为各种化学和电子应用编程。这些材料的成功取决于对分子间相互作用进行编程以指导精确结构排序的能力。为实现这一目标,我们设计并合成了一系列基于双(三唑)苯的 π 共轭分子。我们的合成利用最后阶段的 CC 交叉偶联反应将锯齿形线性前体封闭为环化产物,因此可以直接并排比较它们在表面的结构依赖性自组装行为和响应外部刺激。确实,扫描隧道显微镜 (STM) 分析显示出明显的差异,因为分子主链的构象灵活性和外围基团的化学结构各不相同。具体而言,烷基链吸附并形成叉指结构,而低聚乙二醇 (OEG) 链保持解吸状态,从而将自组装转移到更密集的 π 共轭核。虽然大环能立即自组装,但它们的线性前体表现出较慢的自组装动力学,这可能归因于构象自由度的差异。我们还发现 STM 尖端的扰动和 cosolutes 的添加深刻影响了自组装和表面图案化的动力学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号