当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dinardokanshones C–E, isonardoeudesmols A–D and nardoeudesmol D from Nardostachys jatamansi DC.
Phytochemistry ( IF 3.2 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.phytochem.2018.03.002 Hong-Hua Wu , Xu Deng , Hu Zhang , Ying-Peng Chen , Shu-Song Ying , Yi-Jing Wu , Yan-Ting Liu , Yan Zhu , Xiu-Mei Gao , Yan-Tong Xu , Li Li
Phytochemistry ( IF 3.2 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.phytochem.2018.03.002 Hong-Hua Wu , Xu Deng , Hu Zhang , Ying-Peng Chen , Shu-Song Ying , Yi-Jing Wu , Yan-Ting Liu , Yan Zhu , Xiu-Mei Gao , Yan-Tong Xu , Li Li
|
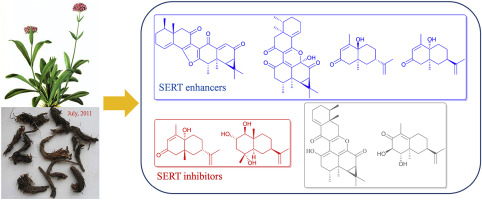
Dinardokanshones C-E, three sesquiterpenoid dimers comprising an unusual nornardosinane-type sesquiterpenoid core and an aristolane-type sesquiterpenoid unit conjugated by an extra pyran or furan ring, together with monomeric sesquiterpenoids isonardoeudesmols A-D and nardoeudesmol D, were isolated from the underground parts of Nardostachys jatamansi DC. Structures of the eight compounds were elucidated by analysis of the extensive spectroscopic data, and their absolute configurations were established by analysis of NOESY and X-ray diffraction data, combined with computational electronic circular dichroism (ECD) calculations. The results of SERT activity assay revealed that isonardoeudesmol D and nardoeudesmol D significantly inhibited SERT activity, while dinardokanshones D-E and isonardoeudesmols B-C significantly enhanced SERT activity, among which dinardokanshone D exhibited the strongest effect.
中文翻译:

Dinardokanshones C–E,来自 Nardostachys jatamansi DC 的 isonardoeudesmols A–D 和 nardoeudesmol D。
Dinardokanshones CE,三个倍半萜类二聚体,包括一个不寻常的去甲萘烷型倍半萜类核心和一个由额外的吡喃或呋喃环共轭的马兜铃型倍半萜类单元,以及单体倍半萜类异松果胶 AD 和 nardoeudesmol D 的地下部分。 . 通过对大量光谱数据的分析阐明了这八种化合物的结构,并通过对 NOESY 和 X 射线衍射数据的分析,结合计算电子圆二色性 (ECD) 计算确定了它们的绝对构型。SERT活性测定结果表明,isoardoeudesmol D和nardoeudesmol D显着抑制SERT活性,而dinardokanshones DE和isoardoeudesmols BC显着增强SERT活性,
更新日期:2018-06-01
中文翻译:

Dinardokanshones C–E,来自 Nardostachys jatamansi DC 的 isonardoeudesmols A–D 和 nardoeudesmol D。
Dinardokanshones CE,三个倍半萜类二聚体,包括一个不寻常的去甲萘烷型倍半萜类核心和一个由额外的吡喃或呋喃环共轭的马兜铃型倍半萜类单元,以及单体倍半萜类异松果胶 AD 和 nardoeudesmol D 的地下部分。 . 通过对大量光谱数据的分析阐明了这八种化合物的结构,并通过对 NOESY 和 X 射线衍射数据的分析,结合计算电子圆二色性 (ECD) 计算确定了它们的绝对构型。SERT活性测定结果表明,isoardoeudesmol D和nardoeudesmol D显着抑制SERT活性,而dinardokanshones DE和isoardoeudesmols BC显着增强SERT活性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号