当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Template-Independent Enzymatic Oligonucleotide Synthesis (TiEOS): Its History, Prospects, and Challenges
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.biochem.7b00937 Michael A. Jensen 1 , Ronald W. Davis 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.biochem.7b00937 Michael A. Jensen 1 , Ronald W. Davis 1, 2
Affiliation
|
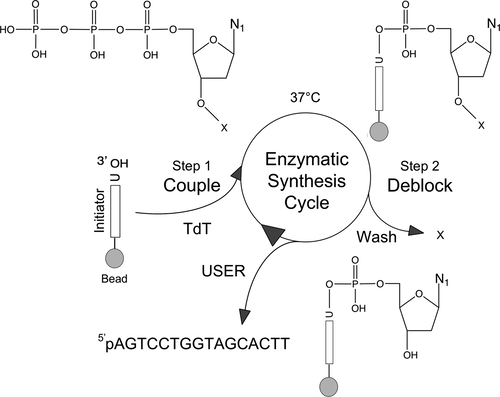
There is a growing demand for sustainable methods in research and development, where instead of hazardous chemicals, an aqueous medium is chosen to perform biological reactions. In this Perspective, we examine the history and current methodology of using enzymes to generate artificial single-stranded DNA. By using traditional solid-phase phosphoramidite chemistry as a metric, we also explore criteria for the method of template-independent enzymatic oligonucleotide synthesis (TiEOS). As its key component, we delve into the biology of one of the most enigmatic enzymes, terminal deoxynucleotidyl transferase (TdT). As TdT is found to exponentially increase antigen receptor diversity in the vertebrate immune system by adding nucleotides in a template-free manner, researchers have exploited this function as an alternative to the phosphoramidite synthesis method. Though TdT is currently the preferred enzyme for TiEOS, its random nucleotide incorporation presents a barrier in synthesis automation. Taking a closer look at the TiEOS cycle, particularly the coupling step, we find it is comprised of additions > n+1 and deletions. By tapping into the physical and biochemical properties of TdT, we strive to further elucidate its mercurial behavior and offer ways to better optimize TiEOS for production-grade oligonucleotide synthesis.
中文翻译:

独立于模板的酶促寡核苷酸合成(TiEOS):其历史,前景和挑战
对研发中可持续方法的需求日益增长,在该方法中,选择了一种水介质而不是危险化学品来进行生物反应。在此透视图中,我们研究了使用酶生成人工单链DNA的历史和当前方法。通过使用传统的固相亚磷酰胺化学方法作为度量标准,我们还探索了独立于模板的酶促寡核苷酸合成方法(TiEOS)的标准。作为其关键组成部分,我们研究了最神秘的酶之一的末端脱氧核苷酸转移酶(TdT)的生物学特性。由于发现TdT通过以无模板的方式添加核苷酸以指数方式增加脊椎动物免疫系统中的抗原受体多样性,研究人员已经将此功能用作亚磷酰胺合成方法的替代方法。尽管TdT目前是TiEOS的首选酶,但其随机核苷酸掺入为合成自动化带来了障碍。仔细观察TiEOS循环,特别是耦合步骤,我们发现它由> n + 1的加法和缺失组成。通过利用TdT的物理和生化特性,我们致力于进一步阐明其汞特性,并提供更好地优化TiEOS的生产级寡核苷酸合成方法。
更新日期:2018-03-13
中文翻译:

独立于模板的酶促寡核苷酸合成(TiEOS):其历史,前景和挑战
对研发中可持续方法的需求日益增长,在该方法中,选择了一种水介质而不是危险化学品来进行生物反应。在此透视图中,我们研究了使用酶生成人工单链DNA的历史和当前方法。通过使用传统的固相亚磷酰胺化学方法作为度量标准,我们还探索了独立于模板的酶促寡核苷酸合成方法(TiEOS)的标准。作为其关键组成部分,我们研究了最神秘的酶之一的末端脱氧核苷酸转移酶(TdT)的生物学特性。由于发现TdT通过以无模板的方式添加核苷酸以指数方式增加脊椎动物免疫系统中的抗原受体多样性,研究人员已经将此功能用作亚磷酰胺合成方法的替代方法。尽管TdT目前是TiEOS的首选酶,但其随机核苷酸掺入为合成自动化带来了障碍。仔细观察TiEOS循环,特别是耦合步骤,我们发现它由> n + 1的加法和缺失组成。通过利用TdT的物理和生化特性,我们致力于进一步阐明其汞特性,并提供更好地优化TiEOS的生产级寡核苷酸合成方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号