当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Hydrocarbon Functionalization by an Iodate/Chloride System: The Role of Ester Protection
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acscatal.7b04397 Nichole A. Schwartz 1 , Nicholas C. Boaz 2 , Steven E. Kalman 1 , Thompson Zhuang 2 , Jonathan M. Goldberg 2 , Ross Fu 3 , Robert J. Nielsen 3 , William A. Goddard 3 , John T. Groves 2 , T. Brent Gunnoe 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acscatal.7b04397 Nichole A. Schwartz 1 , Nicholas C. Boaz 2 , Steven E. Kalman 1 , Thompson Zhuang 2 , Jonathan M. Goldberg 2 , Ross Fu 3 , Robert J. Nielsen 3 , William A. Goddard 3 , John T. Groves 2 , T. Brent Gunnoe 1
Affiliation
|
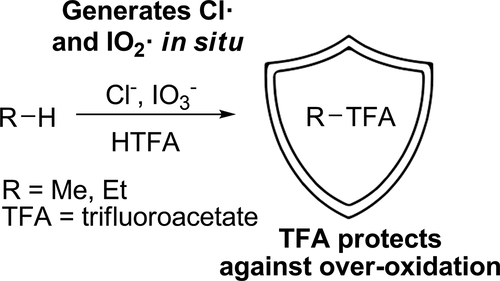
Mixtures of chloride and iodate salts for light alkane oxidation achieve >20% yield of methyl trifluoroacetate (TFA) from methane with >85% selectivity. The mechanism of this C–H oxygenation has been probed by examining adamantane as a model substrate. These recent results lend support to the involvement of free radicals. Comparative studies between radical chlorination and iodate/chloride functionalization of adamantane afford statistically identical 3°:2° selectivities (∼5.2:1) and kinetic isotope effects for C–H/C–D functionalization (kH/kD = 1.6(3), 1.52(3)). Alkane functionalization by iodate/chloride in HTFA is proposed to occur through H-atom abstraction by free radical species including Cl• to give alkyl radicals. Iodine, which forms by in situ reduction of iodate, traps alkyl radicals as alkyl iodides that are subsequently converted to alkyl esters in HTFA solvent. Importantly, the alkyl ester products (RTFA) are quite stable to further oxidation under the oxidizing conditions due to the protecting nature of the ester moiety.
中文翻译:

碘化物/氯化物系统将烃官能化的机理:酯保护的作用
用于轻链烷烃氧化的氯化物和碘酸盐的混合物可从甲烷中获得> 20%的三氟乙酸甲酯(TFA)收率,且具有> 85%的选择性。通过检测金刚烷作为模型底物,已经探究了这种CHH氧化的机理。这些最新结果为自由基的参与提供了支持。自由基氯化和金刚烷碘酸盐/氯化物官能化之间的比较研究提供了统计上相同的3°:2°选择性(〜5.2:1)和C–H / C–D功能化的动力学同位素效应(k H / k D = 1.6(3 ),1.52(3))。HTFA中碘酸盐/氯化物对烷烃的官能化被认为是通过自由基原子(包括Cl •产生烷基。通过碘酸盐原位还原形成的碘将烷基自由基捕获为烷基自由基,随后在HTFA溶剂中将其转化为烷基酯。重要的是,由于酯部分的保护性质,烷基酯产物(RTFA)对于在氧化条件下的进一步氧化是相当稳定的。
更新日期:2018-03-13
中文翻译:

碘化物/氯化物系统将烃官能化的机理:酯保护的作用
用于轻链烷烃氧化的氯化物和碘酸盐的混合物可从甲烷中获得> 20%的三氟乙酸甲酯(TFA)收率,且具有> 85%的选择性。通过检测金刚烷作为模型底物,已经探究了这种CHH氧化的机理。这些最新结果为自由基的参与提供了支持。自由基氯化和金刚烷碘酸盐/氯化物官能化之间的比较研究提供了统计上相同的3°:2°选择性(〜5.2:1)和C–H / C–D功能化的动力学同位素效应(k H / k D = 1.6(3 ),1.52(3))。HTFA中碘酸盐/氯化物对烷烃的官能化被认为是通过自由基原子(包括Cl •产生烷基。通过碘酸盐原位还原形成的碘将烷基自由基捕获为烷基自由基,随后在HTFA溶剂中将其转化为烷基酯。重要的是,由于酯部分的保护性质,烷基酯产物(RTFA)对于在氧化条件下的进一步氧化是相当稳定的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号