当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Role of CO2 in the Soft Oxidative Dehydrogenation of Propane over Ceria-Based Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acscatal.7b03805 Ewa Nowicka 1, 2 , Christian Reece 1 , Sultan M. Althahban 3 , Khaled M. H. Mohammed 4, 5, 6 , Simon A. Kondrat 1, 7 , David J. Morgan 1 , Qian He 1 , David J. Willock 1 , Stanislaw Golunski 1 , Christopher J. Kiely 1, 3 , Graham J. Hutchings 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acscatal.7b03805 Ewa Nowicka 1, 2 , Christian Reece 1 , Sultan M. Althahban 3 , Khaled M. H. Mohammed 4, 5, 6 , Simon A. Kondrat 1, 7 , David J. Morgan 1 , Qian He 1 , David J. Willock 1 , Stanislaw Golunski 1 , Christopher J. Kiely 1, 3 , Graham J. Hutchings 1
Affiliation
|
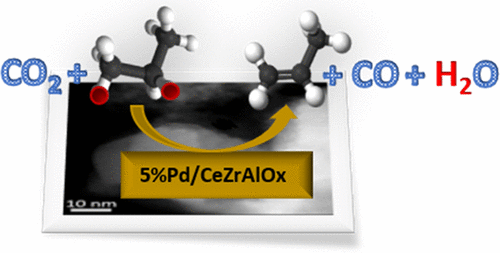
A mixed oxide support containing Ce, Zr, and Al was synthesized using a physical grinding method and applied in the oxidative dehydrogenation of propane using CO2 as the oxidant. The activity of the support was compared with that of fully formulated catalysts containing palladium. The Pd/CeZrAlOx material exhibited long-term stability and selectivity to propene (during continuous operation for 140 h), which is not normally associated with dehydrogenation catalysts. From temperature-programmed desorption of NH3 and CO2 it was found that the catalyst possessed both acidic and basic sites. In addition, temperature-programmed reduction showed that palladium promoted both the reduction and reoxidation of the support. When the role of CO2 was investigated in the absence of gas-phase oxidant, using a temporal analysis of products (TAP) reactor, it was found that CO2 dissociates over the reduced catalyst, leading to formation of CO and selective oxygen species. It is proposed that CO2 has the dual role of regenerating selective oxygen species and shifting the equilibrium for alkane dehydrogenation by consuming H2 through the reverse water-gas-shift reaction. These two mechanistic functions have previously been considered to be mutually exclusive.
中文翻译:

阐明CO 2在二氧化铈基催化剂上丙烷的软氧化脱氢中的作用
使用物理研磨方法合成包含Ce,Zr和Al的混合氧化物载体,并使用CO 2作为氧化剂将其应用于丙烷的氧化脱氢。将载体的活性与含钯的完全配制的催化剂的活性进行了比较。将Pd / CeZrAlO X物质表现出长期稳定性和选择性丙烯,其通常不与脱氢催化剂相关联(140 h持续操作过程中)。通过程序升温的NH 3和CO 2解吸,发现该催化剂同时具有酸性和碱性位点。此外,程序升温还原显示钯促进了载体的还原和再氧化。当CO的作用使用产物的时间分析(TAP)反应器,在不存在气相氧化剂的条件下研究了图2,发现CO 2在还原的催化剂上解离,导致形成CO和选择性氧。提出CO 2具有通过逆水煤气变换反应消耗H 2来再生选择性氧种类和改变烷烃脱氢平衡的双重作用。这两个机械功能以前被认为是互斥的。
更新日期:2018-03-13
中文翻译:

阐明CO 2在二氧化铈基催化剂上丙烷的软氧化脱氢中的作用
使用物理研磨方法合成包含Ce,Zr和Al的混合氧化物载体,并使用CO 2作为氧化剂将其应用于丙烷的氧化脱氢。将载体的活性与含钯的完全配制的催化剂的活性进行了比较。将Pd / CeZrAlO X物质表现出长期稳定性和选择性丙烯,其通常不与脱氢催化剂相关联(140 h持续操作过程中)。通过程序升温的NH 3和CO 2解吸,发现该催化剂同时具有酸性和碱性位点。此外,程序升温还原显示钯促进了载体的还原和再氧化。当CO的作用使用产物的时间分析(TAP)反应器,在不存在气相氧化剂的条件下研究了图2,发现CO 2在还原的催化剂上解离,导致形成CO和选择性氧。提出CO 2具有通过逆水煤气变换反应消耗H 2来再生选择性氧种类和改变烷烃脱氢平衡的双重作用。这两个机械功能以前被认为是互斥的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号