当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective Molecular Catalysts for the CO2-to-CO Electrochemical Conversion at Very Low Overpotential. Contrasting Fe vs Co Quaterpyridine Complexes upon Mechanistic Studies
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acscatal.7b04412 Claudio Cometto 1 , Lingjing Chen 2 , Po-Kam Lo 3 , Zhenguo Guo 3 , Kai-Chung Lau 3 , Elodie Anxolabéhère-Mallart 1 , Claire Fave 1 , Tai-Chu Lau 3 , Marc Robert 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acscatal.7b04412 Claudio Cometto 1 , Lingjing Chen 2 , Po-Kam Lo 3 , Zhenguo Guo 3 , Kai-Chung Lau 3 , Elodie Anxolabéhère-Mallart 1 , Claire Fave 1 , Tai-Chu Lau 3 , Marc Robert 1
Affiliation
|
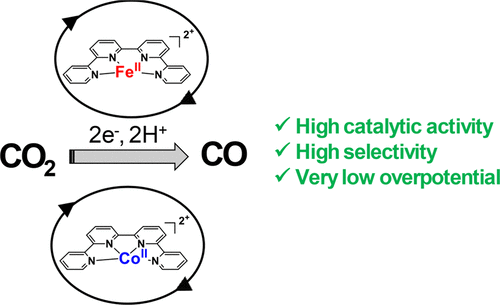
[MII(qpy)(H2O)2]2+ (M = Fe, Co; qpy: 2,2′:6′,2″:6″,2‴-quaterpyridine) complexes efficiently catalyze the electrochemical CO2-to-CO conversion in acetonitrile solution in the presence of weak Brönsted acids. Upon performing cyclic voltammetry studies, controlled-potential electrolysis, and spectroelectrochemistry (UV–visible and infrared) experiments together with DFT calculations, catalytic mechanisms were deciphered. Catalysis is characterized by high selectivity for CO production (selectivity >95%) in the presence of phenol as proton source. Overpotentials as low as 240 and 140 mV for the Fe and Co complexes, respectively, led to large CO production for several hours. In the former case, the one-electron-reduced species binds to CO2, and CO evolution is observed after further reduction of the intermediate adduct. A deactivation pathway has been identified, which is due to the formation of a Fe0qpyCO species. With the Co catalyst, no such deactivation occurs, and the doubly reduced complex activates CO2. High scan rate cyclic voltammetry allows reaching kinetic conditions, leading to scan-rate-independent plateau-shaped voltammograms from which catalytic rate constant was obtained. The molecular catalyst is very active for CO production (turnover a frequency of 3.3 × 104 s–1 at 0.3 V overpotential), as confirmed by catalytic a Tafel plot showing a comparison with previous catalysts.
中文翻译:

在非常低的超电势下用于CO 2到CO电化学转化的高选择性分子催化剂。机理研究对比Fe与Co Quetrpyridine配合物
[M II(qpy)(H 2 O)2 ] 2+(M = Fe,Co; qpy:2,2':6',2″:6″,2‴-四吡啶)络合物有效地催化电化学CO 2在弱布朗斯台德酸的存在下,在乙腈溶液中从CO转化为CO。在进行循环伏安法研究,控制电位电解和光谱电化学(紫外-可见和红外)实验以及DFT计算后,对催化机理进行了解释。在苯酚作为质子源的情况下,催化的特点是对CO的生产具有很高的选择性(选择性> 95%)。Fe和Co络合物的低电势分别低至240和140 mV,导致大量的CO产生持续数小时。在前一种情况下,单电子还原的物质与CO 2结合,并且在进一步还原中间加合物后观察到CO放出。已经确定了失活途径,这是由于形成了Fe 0qpyCO种类。使用Co催化剂时,不会发生这种失活,并且双还原的络合物会活化CO 2。高扫描速率循环伏安法可以达到动力学条件,从而产生不依赖于扫描速率的平台形伏安图,从中可以得到催化速率常数。分子催化剂对一氧化碳生产非常活跃(在0.3 V超电势下,转换频率为3.3×10 4 s –1),通过催化塔菲尔图可知,该催化剂与以前的催化剂进行了比较。
更新日期:2018-03-13
中文翻译:

在非常低的超电势下用于CO 2到CO电化学转化的高选择性分子催化剂。机理研究对比Fe与Co Quetrpyridine配合物
[M II(qpy)(H 2 O)2 ] 2+(M = Fe,Co; qpy:2,2':6',2″:6″,2‴-四吡啶)络合物有效地催化电化学CO 2在弱布朗斯台德酸的存在下,在乙腈溶液中从CO转化为CO。在进行循环伏安法研究,控制电位电解和光谱电化学(紫外-可见和红外)实验以及DFT计算后,对催化机理进行了解释。在苯酚作为质子源的情况下,催化的特点是对CO的生产具有很高的选择性(选择性> 95%)。Fe和Co络合物的低电势分别低至240和140 mV,导致大量的CO产生持续数小时。在前一种情况下,单电子还原的物质与CO 2结合,并且在进一步还原中间加合物后观察到CO放出。已经确定了失活途径,这是由于形成了Fe 0qpyCO种类。使用Co催化剂时,不会发生这种失活,并且双还原的络合物会活化CO 2。高扫描速率循环伏安法可以达到动力学条件,从而产生不依赖于扫描速率的平台形伏安图,从中可以得到催化速率常数。分子催化剂对一氧化碳生产非常活跃(在0.3 V超电势下,转换频率为3.3×10 4 s –1),通过催化塔菲尔图可知,该催化剂与以前的催化剂进行了比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号