当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper catalyzed cyanation through CC bond cleavage of gem-aryl dibromide followed by second cyanation of iodoarene by a released CN unit†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1039/c8qo00108a Pintu Maity 1, 2, 3, 4 , Debasish Kundu 1, 2, 3, 4 , Tubai Ghosh 1, 2, 3, 4 , Brindaban C. Ranu 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1039/c8qo00108a Pintu Maity 1, 2, 3, 4 , Debasish Kundu 1, 2, 3, 4 , Tubai Ghosh 1, 2, 3, 4 , Brindaban C. Ranu 1, 2, 3, 4
Affiliation
|
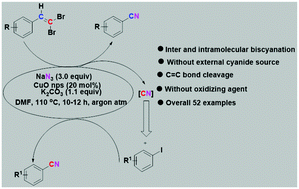
A new approach for the synthesis of aryl cyanides through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C cleavage of styrenyl gem-dibromide via an in situ cyanation process by sodium azide in the presence of CuO nanoparticles and simultaneous intermolecular cyanation of an iodoarene by capturing a released CN unit has been achieved. Several diversely substituted aryl/heteroaryl cyanides including a precursor to etravirine, a drug for the treatment of HIV, have been obtained by this procedure. This protocol is unique with regard to cyanation without a cyanide reagent and provides access to two different nitriles in one reaction involving full utilization of CN units.
C cleavage of styrenyl gem-dibromide via an in situ cyanation process by sodium azide in the presence of CuO nanoparticles and simultaneous intermolecular cyanation of an iodoarene by capturing a released CN unit has been achieved. Several diversely substituted aryl/heteroaryl cyanides including a precursor to etravirine, a drug for the treatment of HIV, have been obtained by this procedure. This protocol is unique with regard to cyanation without a cyanide reagent and provides access to two different nitriles in one reaction involving full utilization of CN units.
中文翻译:

至C铜催化氰化![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 的C键裂解宝石-芳基二溴化物,接着通过释放CN单元iodoarene的第二氰化†
的C键裂解宝石-芳基二溴化物,接着通过释放CN单元iodoarene的第二氰化†
对于芳基氰化物至C合成的新方法![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 苯乙烯的Ç切割宝石-dibromide经由一个原位氰化过程以CuO纳米颗粒和通过捕获释放CN单元的iodoarene同时分子间氰化的存在下由叠氮化钠已经实现。通过这种方法已经获得了几种不同取代的芳基/杂芳基氰化物,包括埃托韦林(一种用于治疗HIV的药物)的前体。对于没有氰化物试剂的氰化,该方案是唯一的,并且在涉及CN单元充分利用的一个反应中提供了进入两个不同腈的途径。
苯乙烯的Ç切割宝石-dibromide经由一个原位氰化过程以CuO纳米颗粒和通过捕获释放CN单元的iodoarene同时分子间氰化的存在下由叠氮化钠已经实现。通过这种方法已经获得了几种不同取代的芳基/杂芳基氰化物,包括埃托韦林(一种用于治疗HIV的药物)的前体。对于没有氰化物试剂的氰化,该方案是唯一的,并且在涉及CN单元充分利用的一个反应中提供了进入两个不同腈的途径。
更新日期:2018-03-13
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C cleavage of styrenyl gem-dibromide via an in situ cyanation process by sodium azide in the presence of CuO nanoparticles and simultaneous intermolecular cyanation of an iodoarene by capturing a released CN unit has been achieved. Several diversely substituted aryl/heteroaryl cyanides including a precursor to etravirine, a drug for the treatment of HIV, have been obtained by this procedure. This protocol is unique with regard to cyanation without a cyanide reagent and provides access to two different nitriles in one reaction involving full utilization of CN units.
C cleavage of styrenyl gem-dibromide via an in situ cyanation process by sodium azide in the presence of CuO nanoparticles and simultaneous intermolecular cyanation of an iodoarene by capturing a released CN unit has been achieved. Several diversely substituted aryl/heteroaryl cyanides including a precursor to etravirine, a drug for the treatment of HIV, have been obtained by this procedure. This protocol is unique with regard to cyanation without a cyanide reagent and provides access to two different nitriles in one reaction involving full utilization of CN units.
中文翻译:

至C铜催化氰化
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 的C键裂解宝石-芳基二溴化物,接着通过释放CN单元iodoarene的第二氰化†
的C键裂解宝石-芳基二溴化物,接着通过释放CN单元iodoarene的第二氰化†
对于芳基氰化物至C合成的新方法
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 苯乙烯的Ç切割宝石-dibromide经由一个原位氰化过程以CuO纳米颗粒和通过捕获释放CN单元的iodoarene同时分子间氰化的存在下由叠氮化钠已经实现。通过这种方法已经获得了几种不同取代的芳基/杂芳基氰化物,包括埃托韦林(一种用于治疗HIV的药物)的前体。对于没有氰化物试剂的氰化,该方案是唯一的,并且在涉及CN单元充分利用的一个反应中提供了进入两个不同腈的途径。
苯乙烯的Ç切割宝石-dibromide经由一个原位氰化过程以CuO纳米颗粒和通过捕获释放CN单元的iodoarene同时分子间氰化的存在下由叠氮化钠已经实现。通过这种方法已经获得了几种不同取代的芳基/杂芳基氰化物,包括埃托韦林(一种用于治疗HIV的药物)的前体。对于没有氰化物试剂的氰化,该方案是唯一的,并且在涉及CN单元充分利用的一个反应中提供了进入两个不同腈的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号