Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-03-13 , DOI: 10.1016/j.actbio.2018.03.013 Zhiqi Xie , Wangwei Guo , Ningning Guo , Mingyi Huangfu , Huina Liu , Mengting Lin , WenHong Xu , Jiejian Chen , TianTian Wang , Qichun Wei , Min Han , Jianqing Gao
|
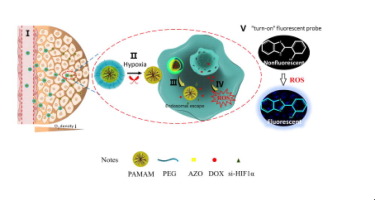
Although existing nanomedicines have focused on the tumor microenvironment with the goal of improving the effectiveness of conventional chemotherapy, the penetration of a tumor’s core still represents a formidable barrier for existing drug delivery systems. Therefore, a novel multifunctional hypoxia-induced size-shrinkable nanoparticle has been designed to increase the penetration of drugs, nucleic acids, or probes into tumors. This cooperative strategy relies on three aspects: (i) the responsiveness of nanoparticles to hypoxia, which shrink when triggered by low oxygen concentrations; (ii) the core of a nanoparticle involves an internal cavity and strong positive charges on the surface to deliver both doxorubicin and siRNA; and (iii) a reactive oxygen species (ROS) probe is incorporated in the nanoparticle to monitor its preliminary therapeutic response in real time, which is expected to realize the enhanced efficacy together with the ability to self-monitor the anticancer activity. A more effective inhibition of tumor growth was observed in tumor-bearing zebrafish, demonstrating the feasibility of this cooperative strategy for in vivo applications. This research highlights a promising value in delivering drugs, nucleic acids, or probes to a tumor’s core for cancer imaging and treatment.
Statement of significance
Hypoxia-induced chemoresistance of tumor cells still represents a formidable barrier, as it is difficult for existing drug delivery systems to penetrate the tumor hypoxia core. This study involves the hypoxia-responsive size-shrinkable nanoparticle co-delivery of DOX and siRNA to enhance the penetration of DOX deep within tumors and subsequently disturb crucial pathways of cancer development induced by hypoxia and to improve sensitization to DOX chemotherapy. Furthermore, the nanopreparation can combine the ROS probe as a self-reporting nanopreparation to realize the function of real-time feedback efficacy, which has a good application prospect in the diagnosis and treatment of cancer.
中文翻译:

使用刺激反应性纳米载体靶向肿瘤缺氧,以克服耐药性并监测抗癌功效
尽管现有的纳米药物已经集中于肿瘤微环境,目的是提高常规化学疗法的有效性,但肿瘤核心的渗透仍然是现有药物输送系统的巨大障碍。因此,已经设计了新颖的多功能缺氧诱导的尺寸可收缩的纳米颗粒,以增加药物,核酸或探针对肿瘤的渗透。这种合作策略依赖于三个方面:(i)纳米颗粒对缺氧的反应性,当低氧浓度触发时,其反应性会降低;(ii)纳米粒子的核心涉及一个内部空腔,并且在表面上具有强正电荷,以同时传递阿霉素和siRNA;(iii)将活性氧(ROS)探针掺入纳米颗粒中以实时监测其初步治疗反应,这有望实现增强的功效以及自我监测抗癌活性的能力。在荷瘤斑马鱼中观察到了更有效的肿瘤生长抑制,证明了这种合作策略在体内应用的可行性。这项研究突显了在将药物,核酸或探针递送到肿瘤核心以进行癌症成像和治疗方面的有希望的价值。证明了这种合作策略在体内应用的可行性。这项研究突显了在将药物,核酸或探针递送到肿瘤核心以进行癌症成像和治疗方面的有希望的价值。证明了这种合作策略在体内应用的可行性。这项研究突显了在将药物,核酸或探针递送到肿瘤核心以进行癌症成像和治疗方面的有希望的价值。
重要声明
缺氧诱导的肿瘤细胞化学抗性仍然代表着强大的屏障,因为现有的药物输送系统很难穿透肿瘤的缺氧核心。这项研究涉及DOX和siRNA的缺氧反应性大小可收缩的纳米颗粒共同递送,以增强DOX在肿瘤内部的渗透,并随后干扰由缺氧诱导的癌症发展的关键途径,并提高对DOX化疗的敏感性。此外,该纳米制剂可以将ROS探针作为一种自报告的纳米制剂,实现实时反馈功效的功能,在癌症的诊断和治疗中具有良好的应用前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号