Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2018-03-12 , DOI: 10.1016/j.cis.2018.01.002 Jarl B. Rosenholm
|
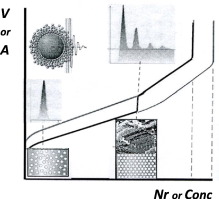
The perfect gas law is used as a reference when selecting state variables (P, V, T, n) needed to characterize ideal gases (vapors), liquids and solids. Van der Waals equation of state is used as a reference for models characterizing interactions in liquids, solids and their mixtures. Van der Waals loop introduces meta- and unstable states between the observed gas (vapor)–liquid P-V transitions at low T. These intermediate states are shown to appear also between liquid–liquid, liquid–solid and solid–solid phase transitions. First-order phase transitions are characterized by a sharp discontinuity of first-order partial derivatives (P, S, V) of Helmholtz and Gibbs free energies. Second-order partial derivatives (KT, B, CV, CP, E) consist of a static contribution relating to second-order phase transitions and a relaxation contribution representing the degree of first-order phase transitions. Bimodal (first-order) and spinodal (second-order) phase boundaries are used to separate stable phases from metastable and unstable phases. The boundaries are identified and quantified by partial derivatives of molar Gibbs free energy or chemical potentials with respect to P, S, V and composition (mole fractions). Molecules confined to spread Langmuir monolayers or adsorbed Gibbs monolayers are characterized by equation of state and adsorption isotherms relating to a two-dimensional van der Waals equation of state. The basic work of two-dimensional wetting (cohesion, adsorption, spreading, immersion), have to be adjusted by a horizontal surface pressure in the presence of adsorbed vapor layers. If the adsorption is extended to liquid films a vertical surface pressure (Π) may be added to account for the lateral interaction, thus restoring PV = ΠAh dependence of thin films. Van der Waals attraction, Coulomb repulsion and structural hydration forces contribute to the vertical surface pressure. A van der Waals type coexistence of ordered (dispersed) and disordered (aggregated) phases is shown to exist when liquid vapor is confined in capillaries (condensation–liquefaction–evaporation and flux). This pheno-menon can be experimentally illustrated with suspended nano-sized particles (flocculation–coagulation–peptisation of colloidal sols) being confined in sample holders of varying size. The self-assembled aggregates represent critical self-similar equilibrium structures corres-ponding to rate determining complexes in kinetics. Overall, a self-consistent thermodynamic framework is established for the characterization of two- and three-dimensional phase separations in one-, two- and three-component systems.
中文翻译:

液体混合物,固体悬浮液和薄膜中范德华型双峰,-λ,-和旋节线相变的表征
选择的状态变量(当理想气体定律被用作参考P,V,T,N )来表征理想气体(蒸气)需要的话,液体和固体。Van der Waals状态方程式用作表征液体,固体及其混合物中相互作用的模型的参考。范德华循环在低T下在观测到的气体(蒸气)-液体P - V跃迁之间引入了亚稳态和不稳定态。这些中间状态也显示在液-液,液-固和固-固相变之间。一阶相变的特征在于一阶偏导数(P,S,V亥姆霍兹和吉布斯自由能的)。二阶偏导数(K T,B,C V,C P,E )由与二阶相变有关的静态贡献和代表一阶相变程度的弛豫贡献组成。双峰(一阶)和旋节线(二阶)相界用于将稳定相与亚稳态相和不稳定相分离。通过摩尔吉布斯自由能或化学势相对于P,S,V的偏导数来识别和量化边界和组成(摩尔分数)。通过状态方程和与二维范德华状态方程有关的吸附等温线来表征局限在朗格缪尔单分子层或吸附的吉布斯单分子层中的分子。二维润湿(内聚,吸附,扩散,浸没)的基本工作必须在存在吸附的蒸汽层的情况下通过水平表面压力进行调整。如果吸附延伸到液体膜的垂直表面压力(Π)可以被添加到帐户的侧向相互作用,从而恢复PV =Π阿薄膜的依赖性。范德华力,库仑排斥力和结构水化力有助于垂直表面压力。当液体蒸汽被限制在毛细管中时(冷凝-液化-蒸发和通量),存在范德华型有序(分散)和无序(聚集)共存的状态。这种现象可通过将纳米级悬浮颗粒(絮凝-凝结-胶态溶胶的胶体化)限制在不同大小的样品架中进行实验说明。自组装聚集体代表了关键的自相似平衡结构,与动力学中的速率决定复合物相对应。总的来说,建立了一个自洽的热力学框架来表征二维和三维一,二和三组分系统中的相分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号