当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Abnormal Tetrel Bonds between Formamidine and TH3F: Substituent Effects
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-13 , DOI: 10.1002/slct.201800025 Huili Xu 1 , Jianbo Cheng 1 , Xuefang Yu 1 , Qingzhong Li 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-13 , DOI: 10.1002/slct.201800025 Huili Xu 1 , Jianbo Cheng 1 , Xuefang Yu 1 , Qingzhong Li 1
Affiliation
|
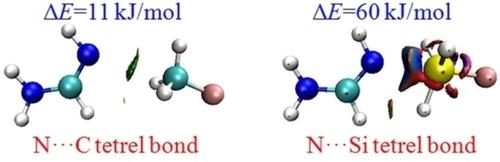
Ab initio calculations have been performed for the complexes of formamidine (FA) with TH3F (T=C, Si, Ge, and Sn) as well as the complexes of FA derivatives including CH3, NH2, OH, OCH3, CH2CH3, CH2CH2CH3, CH(CH3)2, CF3, and F with SiH3F. In general, each complex is stabilized by both a main tetrel bond and a secondary interaction. The stability of the complex increases in the order T=C < Ge < Si < Sn, inconsistent with the magnitude of the σ‐hole on the tetrel atom. The (Z)‐FA complex is more stable than the (E)‐FA analogue. The imino nitrogen atom of FA is a better tetrel acceptor than most nitrogenated bases; it shows a particularly strong affinity for SiH3F with an interaction energy of ∼60 kJ/mol. Four electron‐donating groups give rise to the opposite effect on the strength of the tetrel bond: a weakening effect for OH and OCH3 but an enhancement for CH3 and NH2; the largest interaction energy occurs in CH3‐(Z)‐FA‐SiH3F, amounting to a magnitude of 80 kJ/mol. Two electron‐withdrawing groups have a larger weakening effect than OH and OCH3. The effect of the larger alkyl group is dependent on the type of complex. The weak complexes of CH3F are driven by electrostatic and dispersion interactions, while the strong complexes of TH3F (T=Si, Ge, and Sn) are dominated by electrostatic and polarization interactions, as well as orbital interactions.
中文翻译:

甲am与TH3F之间异常的锡铁键:取代基效应
已对甲am(FA)与TH 3 F(T = C,Si,Ge和Sn)的配合物以及包括CH 3,NH 2,OH,OCH 3在内的FA衍生物的配合物进行了从头算计算。 CH 2 CH 3,CH 2 CH 2 CH 3,CH(CH 3)2,CF 3和含SiH 3的FF.一般而言,每个复合物都通过主要的蝶形键和次要相互作用来稳定。络合物的稳定性按T = C <Ge <Si <Sn的顺序增加,这与铁氧体原子上σ孔的大小不一致。(Z)‐FA复合物比(E)‐FA类似物更稳定。FA的亚氨基氮原子比大多数氮化的碱更好的是铁水星受体。它对SiH 3 F表现出特别强的亲和力,相互作用能约为60 kJ / mol。四个给电子基团对蝶形键的强度产生相反的影响:对OH和OCH 3的减弱作用,对CH 3和NH 2的增强作用。最大的相互作用能发生在CH 3-(Z)-FA-SiH 3中F,总计为80 kJ / mol。两个吸电子基团比OH和OCH 3具有更大的弱化作用。较大烷基的作用取决于配合物的类型。CH 3 F的弱配合物受静电和色散相互作用驱动,而TH 3 F的强配合物(T = Si,Ge和Sn)受静电和极化相互作用以及轨道相互作用控制。
更新日期:2018-03-13
中文翻译:

甲am与TH3F之间异常的锡铁键:取代基效应
已对甲am(FA)与TH 3 F(T = C,Si,Ge和Sn)的配合物以及包括CH 3,NH 2,OH,OCH 3在内的FA衍生物的配合物进行了从头算计算。 CH 2 CH 3,CH 2 CH 2 CH 3,CH(CH 3)2,CF 3和含SiH 3的FF.一般而言,每个复合物都通过主要的蝶形键和次要相互作用来稳定。络合物的稳定性按T = C <Ge <Si <Sn的顺序增加,这与铁氧体原子上σ孔的大小不一致。(Z)‐FA复合物比(E)‐FA类似物更稳定。FA的亚氨基氮原子比大多数氮化的碱更好的是铁水星受体。它对SiH 3 F表现出特别强的亲和力,相互作用能约为60 kJ / mol。四个给电子基团对蝶形键的强度产生相反的影响:对OH和OCH 3的减弱作用,对CH 3和NH 2的增强作用。最大的相互作用能发生在CH 3-(Z)-FA-SiH 3中F,总计为80 kJ / mol。两个吸电子基团比OH和OCH 3具有更大的弱化作用。较大烷基的作用取决于配合物的类型。CH 3 F的弱配合物受静电和色散相互作用驱动,而TH 3 F的强配合物(T = Si,Ge和Sn)受静电和极化相互作用以及轨道相互作用控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号