当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Cleavage of an Asp-Cys Sequence Allows Efficient Production of Recombinant Peptides with an N-Terminal Cysteine Residue
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00083 Katia Pane , Mariavittoria Verrillo 1 , Angela Avitabile , Elio Pizzo , Mario Varcamonti , Anna Zanfardino , Antimo Di Maro 2 , Camilla Rega 2 , Angela Amoresano , Viviana Izzo 3 , Alberto Di Donato , Valeria Cafaro , Eugenio Notomista
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00083 Katia Pane , Mariavittoria Verrillo 1 , Angela Avitabile , Elio Pizzo , Mario Varcamonti , Anna Zanfardino , Antimo Di Maro 2 , Camilla Rega 2 , Angela Amoresano , Viviana Izzo 3 , Alberto Di Donato , Valeria Cafaro , Eugenio Notomista
Affiliation
|
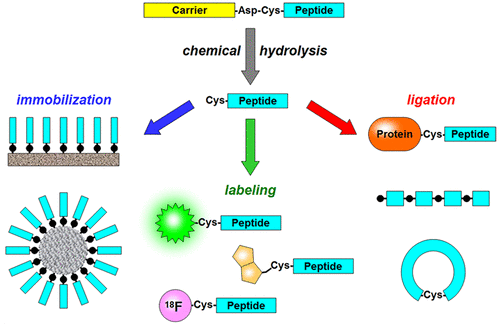
Peptides with an N-terminal cysteine residue allow site-specific modification of proteins and peptides and chemical synthesis of proteins. They have been widely used to develop new strategies for imaging, drug discovery, diagnostics, and chip technologies. Here we present a method to produce recombinant peptides with an N-terminal cysteine residue as a convenient alternative to chemical synthesis. The method is based on the release of the desired peptide from a recombinant fusion protein by mild acid hydrolysis of an Asp-Cys sequence. To test the general validity of the method we prepared four fusion proteins bearing three different peptides (20–37 amino acid long) at the C-terminus of a ketosteroid isomerase-derived and two Onconase-derived carriers for the production of toxic peptides in E. coli. The chosen peptides were (C)GKY20, an antimicrobial peptide from the C-terminus of human thrombin, (C)ApoBL, an antimicrobial peptide from an inner region of human Apolipoprotein B, and (C)p53pAnt, an anticancer peptide containing the C-terminal region of the p53 protein fused to the cell penetrating peptide Penetratin. Cleavage efficiency of Asp-Cys bonds in the four fusion proteins was studied as a function of pH, temperature, and incubation time. In spite of the differences in the amino acid sequence (GTGDCGKY, GTGDCHVA, GSGTDCGSR, SQGSDCGSR) we obtained for all the proteins a cleavage efficiency of about 70–80% after 24 h incubation at 60 °C and pH 2. All the peptides were produced with very good yield (5–16 mg/L of LB cultures), high purity (>96%), and the expected content of free thiol groups (1 mol per mole of peptide). Furthermore, (C)GKY20 was modified with PyMPO-maleimide, a commercially available fluorophore bearing a thiol reactive group, and with 6-hydroxy-2-cyanobenzothiazole, a reagent specific for N-terminal cysteines, with yields of 100% thus demonstrating that our method is very well suited for the production of fully reactive peptides with an N-terminal cysteine residue.
中文翻译:

Asp-Cys序列的化学切割可以高效生产带有N端半胱氨酸残基的重组肽
具有N端半胱氨酸残基的肽可以对蛋白质和肽进行位点特异性修饰,并可以进行蛋白质的化学合成。它们已被广泛用于开发成像,药物发现,诊断和芯片技术的新策略。在这里,我们提出一种生产具有N端半胱氨酸残基的重组肽的方法,以作为化学合成的方便替代方法。该方法基于通过Asp-Cys序列的轻度酸水解从重组融合蛋白中释放所需肽。为了测试该方法的一般有效性,我们制备了四种融合蛋白,它们在酮类固醇异构酶衍生的载体和两种Onconase衍生的载体的C末端带有3个不同的肽(长20-37个氨基酸长),用于在E中产生毒性肽大肠杆菌。选择的肽是(C)GKY20,一种来自人凝血酶C端的抗菌肽,(C)ApoB L,一种来自人载脂蛋白B内部区域的抗菌肽,以及(C)p53pAnt,一种含有抗癌肽的抗癌肽。 p53蛋白的C末端区域与穿透细胞的肽Penetratin融合。研究了四种融合蛋白中Asp-Cys键的切割效率与pH,温度和孵育时间的关系。尽管氨基酸序列有所不同(GTG DC GKY,GTG DC HVA,GSGT DC GSR,SQGS DCGSR),在60°C和pH 2下孵育24小时后,我们对所有蛋白质的切割效率约为70-80%。所有肽的产率都很高(LB培养物为5-16 mg / L),高纯度(> 96%),以及预期的游离硫醇基含量(每摩尔肽1摩尔)。此外,(C)GKY20用PyMPO-马来酰亚胺(带有巯基反应基团的市售荧光团)和6-羟基-2-氰基苯并噻唑(对N端半胱氨酸特异的试剂)进行了修饰,其收率为100%,表明我们的方法非常适合生产具有N端半胱氨酸残基的完全反应性肽。
更新日期:2018-03-12
中文翻译:

Asp-Cys序列的化学切割可以高效生产带有N端半胱氨酸残基的重组肽
具有N端半胱氨酸残基的肽可以对蛋白质和肽进行位点特异性修饰,并可以进行蛋白质的化学合成。它们已被广泛用于开发成像,药物发现,诊断和芯片技术的新策略。在这里,我们提出一种生产具有N端半胱氨酸残基的重组肽的方法,以作为化学合成的方便替代方法。该方法基于通过Asp-Cys序列的轻度酸水解从重组融合蛋白中释放所需肽。为了测试该方法的一般有效性,我们制备了四种融合蛋白,它们在酮类固醇异构酶衍生的载体和两种Onconase衍生的载体的C末端带有3个不同的肽(长20-37个氨基酸长),用于在E中产生毒性肽大肠杆菌。选择的肽是(C)GKY20,一种来自人凝血酶C端的抗菌肽,(C)ApoB L,一种来自人载脂蛋白B内部区域的抗菌肽,以及(C)p53pAnt,一种含有抗癌肽的抗癌肽。 p53蛋白的C末端区域与穿透细胞的肽Penetratin融合。研究了四种融合蛋白中Asp-Cys键的切割效率与pH,温度和孵育时间的关系。尽管氨基酸序列有所不同(GTG DC GKY,GTG DC HVA,GSGT DC GSR,SQGS DCGSR),在60°C和pH 2下孵育24小时后,我们对所有蛋白质的切割效率约为70-80%。所有肽的产率都很高(LB培养物为5-16 mg / L),高纯度(> 96%),以及预期的游离硫醇基含量(每摩尔肽1摩尔)。此外,(C)GKY20用PyMPO-马来酰亚胺(带有巯基反应基团的市售荧光团)和6-羟基-2-氰基苯并噻唑(对N端半胱氨酸特异的试剂)进行了修饰,其收率为100%,表明我们的方法非常适合生产具有N端半胱氨酸残基的完全反应性肽。









































 京公网安备 11010802027423号
京公网安备 11010802027423号