当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-Body Hydrogen Bond Defects Contribute Significantly to the Dielectric Properties of the Liquid Water–Vapor Interface
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.jpclett.8b00488 Sucheol Shin 1 , Adam P. Willard 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.jpclett.8b00488 Sucheol Shin 1 , Adam P. Willard 1
Affiliation
|
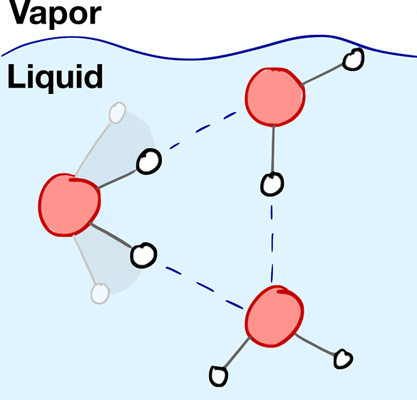
We present a simple model of aqueous interfacial molecular structure, and we use this model to isolate the effects of hydrogen bonding on the dielectric properties of the liquid water–vapor interface. We show that water’s interfacial molecular structure can be understood by considering the orientational preferences of a single molecule immersed in the environment of the average interfacial density field. We illustrate that depth-dependent orientational anisotropy is determined by the geometric constraints of hydrogen bonding, and we show that the primary features of atomistic simulation data can be reproduced by assuming an idealized, perfectly tetrahedral hydrogen bonding geometry. We demonstrate that nonideal hydrogen bond geometries are required to account for variations in the orientational polarization and polarizability of the interface. Finally, we highlight that these properties contain significant contributions from a specific type of geometrically distorted three-body hydrogen bond defect that is preferentially stabilized at the interface.
中文翻译:

三体氢键缺陷对液态水-蒸气界面的介电性能有重大贡献
我们提出了一个简单的水界面分子结构模型,并使用该模型来分离氢键对液态水-蒸汽界面介电性能的影响。我们表明,可以通过考虑浸入平均界面密度场环境中的单个分子的取向偏好来理解水的界面分子结构。我们说明了深度依赖的取向各向异性是由氢键的几何约束决定的,并且我们表明,可以通过假设理想化的,完美的四面体氢键几何形状来再现原子模拟数据的主要特征。我们证明非理想的氢键几何形状是必需的,以解决界面的定向极化和极化率的变化。
更新日期:2018-03-12
中文翻译:

三体氢键缺陷对液态水-蒸气界面的介电性能有重大贡献
我们提出了一个简单的水界面分子结构模型,并使用该模型来分离氢键对液态水-蒸汽界面介电性能的影响。我们表明,可以通过考虑浸入平均界面密度场环境中的单个分子的取向偏好来理解水的界面分子结构。我们说明了深度依赖的取向各向异性是由氢键的几何约束决定的,并且我们表明,可以通过假设理想化的,完美的四面体氢键几何形状来再现原子模拟数据的主要特征。我们证明非理想的氢键几何形状是必需的,以解决界面的定向极化和极化率的变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号