当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Experimental and Computational Investigation of the Elementary Reaction of Ground State Atomic Carbon (C; 3Pj) with Pyridine (C5H5N; X1A1) via Ring Expansion and Ring Degradation Pathways
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.jpca.8b00756 Michael Lucas 1 , Aaron M. Thomas 1 , Ralf I. Kaiser 1 , Eugene K. Bashkirov 2 , Valeriy N. Azyazov 2 , Alexander M. Mebel 2, 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.jpca.8b00756 Michael Lucas 1 , Aaron M. Thomas 1 , Ralf I. Kaiser 1 , Eugene K. Bashkirov 2 , Valeriy N. Azyazov 2 , Alexander M. Mebel 2, 3
Affiliation
|
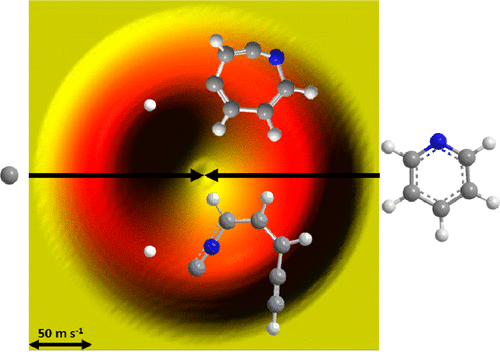
We explored the elementary reaction of atomic carbon (C; 3Pj) with pyridine (C5H5N; X1A1) at a collision energy of 34 ± 4 kJ mol–1 utilizing the crossed molecular beams technique. Forward-convolution fitting of the data was combined with high-level electronic structure calculations and statistical (RRKM) calculations on the triplet C6H5N potential energy surface (PES). These investigations reveal that the reaction dynamics are indirect and dominated by large range reactive impact parameters leading via barrier-less addition to the nitrogen atom and to two chemically nonequivalent “aromatic” carbon–carbon bonds forming three distinct collision complexes. At least two reaction pathways through atomic hydrogen loss were identified on the triplet surface. These channels involve multiple isomerization steps of the initial collision complexes via ring-opening and ring expansion forming an acyclic 1-ethynyl-3-isocyanoallyl radical (P1; 2A″) and a hitherto unreported seven-membered 1-aza-2-dehydrocyclohepta-2,4,6-trien-4-yl radical isomer (P3; 2A), respectively. For RRKM calculations at zero collision energy, representing conditions in cold molecular clouds, the ring expansion product P3 is formed nearly exclusively for the atomic hydrogen loss channel, but based on these computations, the molecular fragmentation channel forming acetylene (C2H2) plus 3-cyano-2-propen-1-ylidene (P6; 3A″) accounts for nearly all of the degradation products of the reaction of atomic carbon with pyridine, proposing a destruction pathway of interstellar pyridine, which may account for the absence in the detection of pyridine in the interstellar medium. These results are also discussed in light of the isoelectronic carbon–benzene (C6H6; X1A1) system with important implications to the rapid degradation of nitrogen-bearing polycyclic aromatic hydrocarbons (NPAHs) in the interstellar medium compared to mass growth processes of PAH counterparts through ring expansion.
中文翻译:

基态原子碳(C; 3 P j)与吡啶(C 5 H 5 N; X 1 A 1)经由环扩环和环降解路径的基础反应的组合实验和计算研究
我们利用交叉分子束技术探索了在34±4 kJ mol –1的碰撞能量下,原子碳(C; 3 P j)与吡啶(C 5 H 5 N; X 1 A 1)的元素反应。数据的正向卷积拟合与三元组C 6 H 5的高级电子结构计算和统计(RRKM)计算相结合N势能面(PES)。这些研究表明,反应动力学是间接的,并受到大范围反应性冲击参数的控制,这些参数主要是通过无障碍添加氮原子并形成两个化学上不等价的“芳族”碳-碳键而形成的,这三个不同的碰撞复合物。在三重态表面上鉴定出至少两个通过原子氢损失的反应途径。这些通道通过开环和扩环形成初始碰撞配合物的多个异构化步骤,形成一个无环的1-乙炔基-3-异氰基烯丙基(P1;2 A”)和迄今未报道的七元1-氮杂-2-脱氢环庚烷-2,4,6-三烯-4-基自由基异构体(P3 ;2 A)。对于零碰撞能量下的RRKM计算(代表冷分子云中的条件),几乎只为原子氢损失通道形成了环膨胀产物P3,但基于这些计算,分子碎片通道形成了乙炔(C 2 H 2)加3-氰基-2-丙烯-1-亚吡咯(P6 ; 3 A'')几乎解释了原子碳与吡啶反应的所有降解产物,提出了星际吡啶的破坏途径,这可能是造成星状吡啶不存在的原因。在星际介质中检测吡啶。还根据等电子碳-苯(C 6高6 ; X 1 A 1)系统对星际介质中含氮多环芳烃(NPAH)的快速降解具有重要意义,与PAH对应物通过扩环的质量增长过程相比。
更新日期:2018-03-12
中文翻译:

基态原子碳(C; 3 P j)与吡啶(C 5 H 5 N; X 1 A 1)经由环扩环和环降解路径的基础反应的组合实验和计算研究
我们利用交叉分子束技术探索了在34±4 kJ mol –1的碰撞能量下,原子碳(C; 3 P j)与吡啶(C 5 H 5 N; X 1 A 1)的元素反应。数据的正向卷积拟合与三元组C 6 H 5的高级电子结构计算和统计(RRKM)计算相结合N势能面(PES)。这些研究表明,反应动力学是间接的,并受到大范围反应性冲击参数的控制,这些参数主要是通过无障碍添加氮原子并形成两个化学上不等价的“芳族”碳-碳键而形成的,这三个不同的碰撞复合物。在三重态表面上鉴定出至少两个通过原子氢损失的反应途径。这些通道通过开环和扩环形成初始碰撞配合物的多个异构化步骤,形成一个无环的1-乙炔基-3-异氰基烯丙基(P1;2 A”)和迄今未报道的七元1-氮杂-2-脱氢环庚烷-2,4,6-三烯-4-基自由基异构体(P3 ;2 A)。对于零碰撞能量下的RRKM计算(代表冷分子云中的条件),几乎只为原子氢损失通道形成了环膨胀产物P3,但基于这些计算,分子碎片通道形成了乙炔(C 2 H 2)加3-氰基-2-丙烯-1-亚吡咯(P6 ; 3 A'')几乎解释了原子碳与吡啶反应的所有降解产物,提出了星际吡啶的破坏途径,这可能是造成星状吡啶不存在的原因。在星际介质中检测吡啶。还根据等电子碳-苯(C 6高6 ; X 1 A 1)系统对星际介质中含氮多环芳烃(NPAH)的快速降解具有重要意义,与PAH对应物通过扩环的质量增长过程相比。











































 京公网安备 11010802027423号
京公网安备 11010802027423号