当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Transannular Approach to Stereodefined Bicyclo[3.1.0]hexanes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.joc.8b00165 Iker Riaño 1 , Uxue Uria 1 , Efraím Reyes 1 , Luisa Carrillo 1 , Jose L. Vicario 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.joc.8b00165 Iker Riaño 1 , Uxue Uria 1 , Efraím Reyes 1 , Luisa Carrillo 1 , Jose L. Vicario 1
Affiliation
|
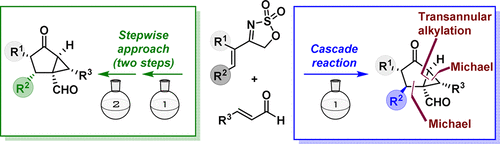
A diastereodivergent approach to highly substituted bicyclo[3.1.0]hexanes has been developed through a transannular alkylation reaction that builds up the bicyclic core employing asymmetric organocatalysis as the tool for the installation of all stereocenters. On one hand, a Michael/Michael cascade process between enals and 4-alkenyl sulfamidate imines under the iminium/enamine activation manifold provides an oxathiazole-2,2-dioxide-fused cyclohexane adduct that, after isolation, is subsequently engaged in a transannular alkylation/hydrolysis through enamine activation by the use of a primary amine. On the other hand, the corresponding C-2 epimers are directly obtained from the same starting materials in a single operation through a cascade Michael/Michael/transannular alkylation/hydrolysis sequence through sequential iminium/enamine/enamine combination of aminocatalytic activation manifolds.
中文翻译:

立体定义双环[3.1.0]己烷的有机催化跨环途径
通过跨环烷基化反应已开发出一种非对映异构的方法,用于高度取代的双环[3.1.0]己烷,该反应使用不对称有机催化作为安装所有立构中心的工具来构建双环核心。一方面,在亚胺/烯胺活化歧管下,烯醛和4-烯基氨基磺酸亚胺之间的迈克尔/迈克尔级联过程提供了氧杂噻唑-2,2-二氧化物-稠合的环己烷加合物,在分离后随后将其进行跨环烷基化通过使用伯胺的烯胺活化进行水解。另一方面,
更新日期:2018-03-12
中文翻译:

立体定义双环[3.1.0]己烷的有机催化跨环途径
通过跨环烷基化反应已开发出一种非对映异构的方法,用于高度取代的双环[3.1.0]己烷,该反应使用不对称有机催化作为安装所有立构中心的工具来构建双环核心。一方面,在亚胺/烯胺活化歧管下,烯醛和4-烯基氨基磺酸亚胺之间的迈克尔/迈克尔级联过程提供了氧杂噻唑-2,2-二氧化物-稠合的环己烷加合物,在分离后随后将其进行跨环烷基化通过使用伯胺的烯胺活化进行水解。另一方面,











































 京公网安备 11010802027423号
京公网安备 11010802027423号