Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2018-03-12 , DOI: 10.1016/j.apcatb.2018.03.037 Seif Yusuf , Luke Neal , Vasudev Haribal , Madison Baldwin , H. Henry Lamb , Fanxing Li
|
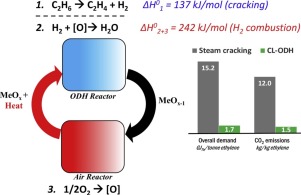
The current study investigates manganese silicate based redox catalysts for ethane to ethylene conversion in a chemical looping oxidative dehydrogenation (CL-ODH) process. Facilitated by a two-step cyclic redox scheme, CL-ODH has the potential to overcome the drawbacks of traditional steam cracking including high energy consumption, coke formation, and significant CO2 and NOx emissions. In CL-ODH, lattice oxygen in manganese silicate based redox catalysts is used to combust the hydrogen formed from ethane dehydrogenation, enhancing ethylene formation and suppressing coke formation. The oxygen-deprived redox catalyst is subsequently regenerated with air, releasing heat to balance the overall heat requirement. The key to this process is an efficient redox catalyst with high selectivity and facile oxygen transport. In this study, redox catalysts with combined manganese and silica phases were tested. We report that redox catalysts with high manganese content are more effective for CL-ODH due to their higher oxygen capacity at reaction temperatures. Sodium tungstate was used as a promoter due to its effectiveness to suppress COx formation. Among the redox catalysts investigated, sodium tungstate promoted (1.7 wt.% Na) manganese silicate (Mn:Si molar ratio = 70:30) was the most effective, showing an ethylene selectivity of 82.6% and yield of 63.3%. Temperature programmed reaction (TPR) experiments indicate that the sodium tungstate promoter inhibits ethane activation on the surface of the redox catalyst and is selective towards hydrogen combustion. XPS analysis indicates that the manganese silicate redox catalysts have a smaller amount of near surface Mn4+ than previously studied manganese containing redox catalysts, leading to higher ethylene selectivity on the un-promoted redox catalysts. XPS also indicates that the reduction of the un-promoted redox catalysts leads to the consumption of silica and formation of inosilicate species. ASPEN Plus® simulations of the CL-ODH scheme using manganese silicate based redox catalysts indicate significant energy and emissions savings compared to traditional steam cracking: the overall energy consumption for ethylene production can potentially be reduced by 89% using the manganese silicate based redox catalyst in the CL-ODH process. Resulting from the significant energy savings, CO2/NOx emissions can be reduced by nearly one order of magnitude when compared to traditional steam cracking.
中文翻译:

硅酸锰基氧化还原催化剂,可通过化学环化生产更绿色的乙烯-乙烷的氧化脱氢
目前的研究是在化学环氧化脱氢(CL-ODH)过程中研究基于硅酸锰的氧化还原催化剂,以将乙烷转化为乙烯。通过两步循环氧化还原方案,CL-ODH有潜力克服传统蒸汽裂解的缺点,包括高能耗,结焦,以及大量的CO 2和NO x。排放。在CL-ODH中,硅酸锰基氧化还原催化剂中的晶格氧用于燃烧乙烷脱氢形成的氢,增强乙烯的形成并抑制焦炭的形成。缺氧的氧化还原催化剂随后与空气再生,释放热量以平衡总热量需求。该方法的关键是高效的氧化还原催化剂,具有高选择性和便捷的氧气传输能力。在这项研究中,测试了具有锰和二氧化硅相的氧化还原催化剂。我们报告说,高锰含量的氧化还原催化剂对CL-ODH更为有效,因为它们在反应温度下的氧气容量更高。钨酸钠由于具有抑制CO x的作用而被用作助催化剂编队。在所研究的氧化还原催化剂中,钨酸钠促进的(1.7重量%Na)硅酸锰锰(Mn:Si摩尔比= 70:30)最为有效,乙烯选择性为82.6%,产率为63.3%。程序升温反应(TPR)实验表明,钨酸钠助催化剂可抑制氧化还原催化剂表面的乙烷活化,并且对氢燃烧具有选择性。XPS分析表明,硅酸锰氧化还原催化剂的近表面Mn 4+含量较小。与先前研究的含锰的氧化还原催化剂相比,它在未促进的氧化还原催化剂上具有更高的乙烯选择性。XPS还表明未促进的氧化还原催化剂的还原会导致二氧化硅的消耗和硅酸盐物种的形成。ASPEN加®使用锰基于硅酸盐的氧化还原催化剂的CL-ODH方案的模拟表明显著能源和排放储蓄相对于传统的蒸汽裂化:可以潜在地通过89%使用基于在氧化还原催化剂的硅酸锰减少用于乙烯生产的总能量消耗CL-ODH过程。由于大量节能,CO 2 / NO x 与传统的蒸汽裂解相比,排放量可减少近一个数量级。



























 京公网安备 11010802027423号
京公网安备 11010802027423号