当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Biological Evaluation, Molecular Docking and DFT Study of Potent Antileishmanial Agents Based on the Thiazolo[3, 2‐a]pyrimidine Chemical Scaffold
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-12 , DOI: 10.1002/slct.201800056 Radha N. Chaturvedi 1, 2 , Mohd Arish 3 , Mohammad Kashif 3, 4 , Varinder Kumar 5 , Reenu 6 , Krishnaiah Pendem 7 , Abdur Rub 3, 8 , Sunita Malhotra 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-12 , DOI: 10.1002/slct.201800056 Radha N. Chaturvedi 1, 2 , Mohd Arish 3 , Mohammad Kashif 3, 4 , Varinder Kumar 5 , Reenu 6 , Krishnaiah Pendem 7 , Abdur Rub 3, 8 , Sunita Malhotra 2
Affiliation
|
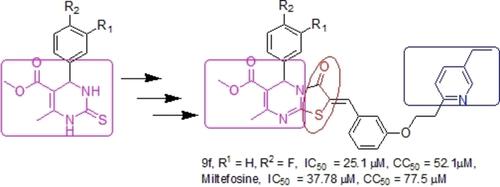
A series of 20 compounds having thiazolo[3, 2‐a]pyrimidine chemical scaffold were synthesized and evaluated for their antileishmanial activity against promastigotes of Leishmania donovani. Amongst all, two compounds showed promising antileishmanial activity in comparison to other compounds. Inhibitory concentration 50% (IC50) was calculated as 42.1 μM and 25.1 μM with selectivity index of 8.3 and 6.05, respectively against Miltefosine (reference drug) 37.78 μM with selectivity index of 2.05. To confirm the target of the these molecules, we modelled Leishmania donovani Ca2+ ion channel (LdCC) protein and performed the docking analysis of the best antileishmanial activity exhibiting inhibitors. The free energy of binding was observed as −10.2 and −9.6 kcal mol−1 in comparison to reference drug −6.2 kcal mol−1. It also makes several hydrogen bonds with our conserved residue Ser1655, Tyr1598 and Asn927. Furthermore, several hydrophobic contacts were also observed within the pocket. Finally, computational work employing density functional theory (DFT) was also carried out to investigate the electronic properties of the synthesized compounds. The in vitro and in silico activities conclusively revealed that our lead compounds may be used as a novel therapeutics against leishmaniasis.
中文翻译:

基于噻唑并[3,2-a]嘧啶化学骨架的强抗leishmanialian剂的合成,生物学评估,分子对接和DFT研究
合成了20种具有噻唑并[3,2- a ]嘧啶化学骨架的化合物,并评估了它们对利什曼原虫的前鞭毛体的抗疟疾活性。在所有化合物中,与其他化合物相比,有两种化合物显示出令人满意的抗菌活性。计算得出的相对于Miltefosine(参考药物)37.78μM,选择性指数为2.05的抑制浓度50%(IC 50)分别为42.1μM和25.1μM,选择性指数为8.3和6.05。为了确认这些分子的靶标,我们对利什曼原虫donovani Ca 2+进行了建模离子通道(LdCC)蛋白,并进行了对表现出最佳抑菌活性的抑制剂的对接分析。观察到结合的自由能为-10.2 -9.6和千卡摩尔-1相较于参考药物-6.2千卡摩尔-1。它还与我们保守的残基Ser1655,Tyr1598和Asn927形成多个氢键。此外,在口袋中还观察到了几个疏水性接触。最后,还利用密度泛函理论(DFT)进行了计算工作,以研究合成化合物的电子性质。体外和计算机活性最终表明,我们的先导化合物可用作抗利什曼病的新型疗法。
更新日期:2018-03-12
中文翻译:

基于噻唑并[3,2-a]嘧啶化学骨架的强抗leishmanialian剂的合成,生物学评估,分子对接和DFT研究
合成了20种具有噻唑并[3,2- a ]嘧啶化学骨架的化合物,并评估了它们对利什曼原虫的前鞭毛体的抗疟疾活性。在所有化合物中,与其他化合物相比,有两种化合物显示出令人满意的抗菌活性。计算得出的相对于Miltefosine(参考药物)37.78μM,选择性指数为2.05的抑制浓度50%(IC 50)分别为42.1μM和25.1μM,选择性指数为8.3和6.05。为了确认这些分子的靶标,我们对利什曼原虫donovani Ca 2+进行了建模离子通道(LdCC)蛋白,并进行了对表现出最佳抑菌活性的抑制剂的对接分析。观察到结合的自由能为-10.2 -9.6和千卡摩尔-1相较于参考药物-6.2千卡摩尔-1。它还与我们保守的残基Ser1655,Tyr1598和Asn927形成多个氢键。此外,在口袋中还观察到了几个疏水性接触。最后,还利用密度泛函理论(DFT)进行了计算工作,以研究合成化合物的电子性质。体外和计算机活性最终表明,我们的先导化合物可用作抗利什曼病的新型疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号