当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cl‐Radical Formation in UV Photodissociation of Tetrachlorocyclopropene: A REMPI Study
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-12 , DOI: 10.1002/slct.201702760 Sumana SenGupta 1 , Ankur Saha 1 , Awadhesh Kumar 1 , Prakash D. Naik 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-12 , DOI: 10.1002/slct.201702760 Sumana SenGupta 1 , Ankur Saha 1 , Awadhesh Kumar 1 , Prakash D. Naik 1
Affiliation
|
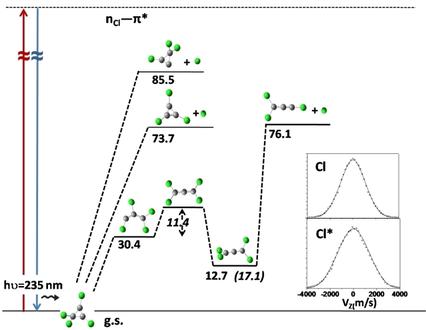
Cl atoms in both ground [Cl(2P3/2)] and excited spin orbit states [Cl*(2P1/2)] were detected as photodissociation products of tetrachlorocyclopropene (TCCP) at 235 nm by Resonance Enhanced Multiphoton Ionisation – Time of Flight (REMPI‐TOF) technique. From the experimental REMPI‐TOF profile, the anisotropy parameter (β) for both the channels was found to be nearly zero. TOF profiles of both the Cl and Cl* channels could be fitted into single component translational energy distributions, with average energy of 7.7 and 8.6 kcal mol−1 respectively. Time Dependent – Density Functional Theory (TD‐DFT) calculations using mpw1pw91/aug‐cc‐pVTZ level of theory suggested that, near 235 nm, the initially excited nCl—π* state of TCCP may cross over to the nearby dissociative states, from where Cl/Cl* can be formed directly. Otherwise, the excited molecules may relax rapidly to the ground state, where the lowest energy dissociation channel is the C—C bond cleavage (ΔH=30.4 kcal mol−1), as C—Cl bond breaking channels need high energy (ΔH=73.7 kcal mol−1 and 85.5 kcal mol−1). The biradical product of C—C bond breaking may rearrange to tetrachloroallene molecule with four equivalent C—Cl bonds, which can subsequently undergo C—Cl bond dissociation to produce Cl/Cl* atoms.
中文翻译:

四氯环丙烯在紫外光解离中的Cl-自由基形成:REMPI研究
通过共振增强多光子电离,检测到基态[Cl(2 P 3/2)]和激发自旋轨道态[Cl *(2 P 1/2)]中的Cl原子是四氯环丙烯(TCCP)在235 nm处的光解产物–飞行时间(REMPI-TOF)技术。从实验的REMPI-TOF剖面图中,发现两个通道的各向异性参数(β)几乎为零。Cl和Cl *通道的TOF分布可以拟合为单组分平移能量分布,平均能量分别为7.7和8.6 kcal mol -1。时间依赖性–使用mpw1pw91 / aug-cc-pVTZ理论水平的密度泛函理论(TD-DFT)计算表明,在235 nm附近,最初激发的nTCCP的Cl- π*状态可能会转变为附近的解离状态,从那里可以直接形成Cl / Cl *。否则,被激发的分子可能会迅速松弛到基态,其中最低的能量解离通道是C键断裂(ΔH= 30.4 kcal mol -1),因为C-Cl键断裂通道需要高能(ΔH= 73.7) kcal mol -1和85.5 kcal mol -1)。CC键断裂的双自由基产物可以重排为具有四个等效CC键的四氯丙烯烯分子,其随后可以经历CC键解离以产生Cl / Cl *原子。
更新日期:2018-03-12
中文翻译:

四氯环丙烯在紫外光解离中的Cl-自由基形成:REMPI研究
通过共振增强多光子电离,检测到基态[Cl(2 P 3/2)]和激发自旋轨道态[Cl *(2 P 1/2)]中的Cl原子是四氯环丙烯(TCCP)在235 nm处的光解产物–飞行时间(REMPI-TOF)技术。从实验的REMPI-TOF剖面图中,发现两个通道的各向异性参数(β)几乎为零。Cl和Cl *通道的TOF分布可以拟合为单组分平移能量分布,平均能量分别为7.7和8.6 kcal mol -1。时间依赖性–使用mpw1pw91 / aug-cc-pVTZ理论水平的密度泛函理论(TD-DFT)计算表明,在235 nm附近,最初激发的nTCCP的Cl- π*状态可能会转变为附近的解离状态,从那里可以直接形成Cl / Cl *。否则,被激发的分子可能会迅速松弛到基态,其中最低的能量解离通道是C键断裂(ΔH= 30.4 kcal mol -1),因为C-Cl键断裂通道需要高能(ΔH= 73.7) kcal mol -1和85.5 kcal mol -1)。CC键断裂的双自由基产物可以重排为具有四个等效CC键的四氯丙烯烯分子,其随后可以经历CC键解离以产生Cl / Cl *原子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号