当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suppressing Li Dendrite Formation in Li2S‐P2S5 Solid Electrolyte by LiI Incorporation
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-03-12 , DOI: 10.1002/aenm.201703644 Fudong Han 1 , Jie Yue 1 , Xiangyang Zhu 1 , Chunsheng Wang 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-03-12 , DOI: 10.1002/aenm.201703644 Fudong Han 1 , Jie Yue 1 , Xiangyang Zhu 1 , Chunsheng Wang 1
Affiliation
|
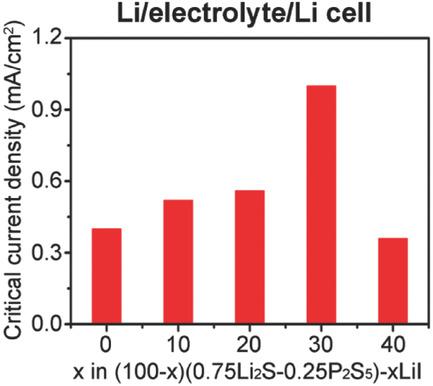
Solid electrolytes have been considered as a promising approach for Li dendrite prevention because of their high mechanical strength and high Li transference number. However, recent reports indicate that Li dendrites also form in Li2S‐P2S5 based sulfide electrolytes at current densities much lower than that in the conventional liquid electrolytes. The methods of suppressing dendrite formation in sulfide electrolytes have rarely been reported because the mechanism for the “unexpected” dendrite formation is unclear, limiting the successful utilization of high‐energy Li anode with these electrolytes. Herein, the authors demonstrate that the Li dendrite formation in Li2S‐P2S5 glass can be effectively suppressed by tuning the composition of the solid electrolyte interphase (SEI) at the Li/electrolyte interface through incorporating LiI into the electrolyte. This approach introduces high ionic conductivity but electronic insulation of LiI in the SEI, and more importantly, improves the mobility of Li atoms, promoting the Li depositon at the interface and thus suppresses dendrite growth. It is shown that the critical current density is improved significantly after incorporating LiI into Li2S‐P2S5 glass, reaching 3.90 mA cm−2 at 100 °C after adding 30 mol% LiI. Stable cycling of the Li‐Li cells for 200 h is also achieved at 1.50 mA cm−2 at 100 °C.
中文翻译:

LiI掺入抑制Li2S-P2S5固体电解质中Li树突的形成
固体电解质由于其高的机械强度和高的Li转移数而被认为是预防Li枝晶的一种有前途的方法。但是,最近的报道表明,Li 2 S-P 2 S 5基硫化物电解质中还会形成Li树枝状晶体,其电流密度远低于常规液体电解质中的电流密度。抑制硫化物电解质中枝晶形成的方法很少见,因为尚不清楚“意外”枝晶形成的机理,限制了高能锂阳极在这些电解质中的成功利用。在本文中,作者证明了Li 2 S-P 2 S 5中的Li枝晶形成。通过将LiI掺入电解质中,可以通过调节Li /电解质界面处的固体电解质中间相(SEI)的成分来有效地抑制玻璃。这种方法引入了高离子电导率,但在SEI中实现了LiI的电子绝缘,更重要的是,它提高了Li原子的迁移率,促进了界面处的Li沉积,从而抑制了树枝状晶体的生长。结果表明,将LiI掺入Li 2 S-P 2 S 5玻璃中后,临界电流密度显着提高,在加入30 mol%LiI后,在100°C时,其临界电流密度达到3.90 mA cm -2。Li-Li电池在100°C下在1.50 mA cm -2时也可以稳定循环200小时。
更新日期:2018-03-12
中文翻译:

LiI掺入抑制Li2S-P2S5固体电解质中Li树突的形成
固体电解质由于其高的机械强度和高的Li转移数而被认为是预防Li枝晶的一种有前途的方法。但是,最近的报道表明,Li 2 S-P 2 S 5基硫化物电解质中还会形成Li树枝状晶体,其电流密度远低于常规液体电解质中的电流密度。抑制硫化物电解质中枝晶形成的方法很少见,因为尚不清楚“意外”枝晶形成的机理,限制了高能锂阳极在这些电解质中的成功利用。在本文中,作者证明了Li 2 S-P 2 S 5中的Li枝晶形成。通过将LiI掺入电解质中,可以通过调节Li /电解质界面处的固体电解质中间相(SEI)的成分来有效地抑制玻璃。这种方法引入了高离子电导率,但在SEI中实现了LiI的电子绝缘,更重要的是,它提高了Li原子的迁移率,促进了界面处的Li沉积,从而抑制了树枝状晶体的生长。结果表明,将LiI掺入Li 2 S-P 2 S 5玻璃中后,临界电流密度显着提高,在加入30 mol%LiI后,在100°C时,其临界电流密度达到3.90 mA cm -2。Li-Li电池在100°C下在1.50 mA cm -2时也可以稳定循环200小时。











































 京公网安备 11010802027423号
京公网安备 11010802027423号