当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Studies on Work Functions of Li+ Ions and Electrons in the Battery Electrode Material LiCoO2: A Thermodynamic Cycle Combining Ionic and Electronic Structure
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-03-12 , DOI: 10.1002/aenm.201703411 Stephan Schuld 1 , René Hausbrand 2 , Mathias Fingerle 2 , Wolfram Jaegermann 2 , Karl-Michael Weitzel 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-03-12 , DOI: 10.1002/aenm.201703411 Stephan Schuld 1 , René Hausbrand 2 , Mathias Fingerle 2 , Wolfram Jaegermann 2 , Karl-Michael Weitzel 1
Affiliation
|
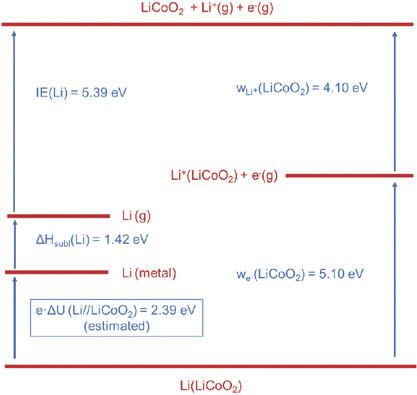
The release of Li+ from stoichiometric LiCoO2 (LCO) – a typical battery electrode material – is investigated by means of thermionic emission. Analysis of the data leads to an ionic work function of wLi+(LCO) = 4.1 eV. Combination of this value with the electronic work function we− (LCO) = 5.1 eV, also measured in this work by photoelectron spectroscopy, and with information available from the literature allows the set up, for the first time, of a complete thermodynamic cycle for a Li//LiCoO2 battery. An open circuit cell voltage of 2.4 eV is derived in line with available literature information. The proof‐of‐principle study presented here provides experimental data on the binding energy values, i.e., chemical potentials, of Li+‐ions and electrons and thus of Li‐atoms in LiCoO2 as a battery cathode and is expected to open access to a better understanding and thus to a better design of battery materials.
中文翻译:

电池电极材料LiCoO2中Li +离子和电子的功函数的实验研究:结合离子和电子结构的热力学循环
通过热电子发射研究了化学计量的LiCoO 2(LCO)(一种典型的电池电极材料)中Li +的释放。数据分析导致离子功函数为w Li +(LCO)= 4.1 eV。该值与电子功函数w e-(LCO)= 5.1 eV的组合,也通过光电子能谱在此功中测量,并结合文献中的可用信息,首次建立了完整的热力学循环用于Li // LiCoO 2电池。根据可用的文献信息得出了2.4 eV的开路电池电压。本文介绍的原理验证研究提供了有关作为电池阴极的LiCoO 2中Li +离子和电子以及因此Li原子的结合能值(即化学势)的实验数据。更好地理解,从而更好地设计电池材料。
更新日期:2018-03-12
中文翻译:

电池电极材料LiCoO2中Li +离子和电子的功函数的实验研究:结合离子和电子结构的热力学循环
通过热电子发射研究了化学计量的LiCoO 2(LCO)(一种典型的电池电极材料)中Li +的释放。数据分析导致离子功函数为w Li +(LCO)= 4.1 eV。该值与电子功函数w e-(LCO)= 5.1 eV的组合,也通过光电子能谱在此功中测量,并结合文献中的可用信息,首次建立了完整的热力学循环用于Li // LiCoO 2电池。根据可用的文献信息得出了2.4 eV的开路电池电压。本文介绍的原理验证研究提供了有关作为电池阴极的LiCoO 2中Li +离子和电子以及因此Li原子的结合能值(即化学势)的实验数据。更好地理解,从而更好地设计电池材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号