Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic approaches for probing amyloid assembly and behaviour
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1039/c7lc01241a Therese W. Herling 1, 2, 3, 4, 5 , Aviad Levin 1, 2, 3, 4, 5 , Kadi L. Saar 1, 2, 3, 4, 5 , Christopher M. Dobson 1, 2, 3, 4, 5 , Tuomas P. J. Knowles 1, 2, 3, 4, 5
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1039/c7lc01241a Therese W. Herling 1, 2, 3, 4, 5 , Aviad Levin 1, 2, 3, 4, 5 , Kadi L. Saar 1, 2, 3, 4, 5 , Christopher M. Dobson 1, 2, 3, 4, 5 , Tuomas P. J. Knowles 1, 2, 3, 4, 5
Affiliation
|
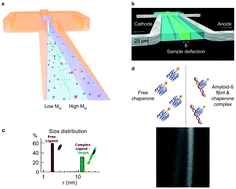
The self-assembly of proteins into supramolecular structures and machinery underpins biological activity in living systems. Misassembled, misfolded and aggregated protein structures can, by contrast, have deleterious activity and such species are at the origin of a number of disease states ranging from cancer to neurodegenerative disorders. In particular, the formation of highly ordered protein aggregates, amyloid fibrils, from normally soluble peptides and proteins, is the common pathological hallmark of a range a group of over fifty protein misfolding disorders. Because of the critical role of the process in the aetiology of such disorders, as well as the quest to understand the basic principles of protein folding and misfolding, the amyloid phenomenon has become a central area of modern biomedical research. Advances in our knowledge of the physical properties of amyloid systems have, however, also highlighted the potential of amyloid structures in the context of materials science. In this review, we explore how microfluidic approaches can be used to study aspects of amyloid assembly and behaviour that are challenging to probe under bulk solution conditions. We discuss the use of volume confinement to probe very early events in the amyloid formation process. In addition, the well-defined fluid flow properties within channels with dimensions on the micron scale can be exploited to measure the physical properties of protein aggregates, such as their sizes and charges, to shed light on the physical and chemical parameters defining amyloid species. Moreover, the molecular species formed during aggregation reactions have physical dimensions spanning at least three orders of magnitude, and microfluidic techniques are well suited to work with analytes of such disparate dimensions. Furthermore, the flexibility of the design of microfluidic devices lends itself to adaptable experimental setups, including the study of protein self-assembly within living cells. Finally, we highlight the salient features of microfluidic experiments that facilitate probing complex biological systems, and discuss their use in the exploration of amyloids as a class of functional material.
中文翻译:

探测淀粉样蛋白组装和行为的微流控方法
蛋白质自组装为超分子结构和机械的基础是活体系统中生物活性的基础。相比之下,错误组装,错误折叠和聚集的蛋白质结构可能具有有害的活性,此类物种是从癌症到神经退行性疾病等多种疾病的起源。特别地,由正常可溶的肽和蛋白质形成高度有序的蛋白质聚集体,淀粉样原纤维是一系列超过五十种蛋白质错误折叠障碍的常见病理学特征。由于该过程在此类疾病的病因学中的关键作用,以及对理解蛋白质折叠和错误折叠的基本原理的追求,淀粉样蛋白现象已成为现代生物医学研究的中心领域。但是,我们对淀粉样蛋白系统物理特性的了解的进步也突出了在材料科学领域中淀粉样蛋白结构的潜力。在这篇综述中,我们探讨了如何使用微流体方法来研究淀粉样蛋白组装和行为方面,这些问题在大体积溶液条件下难以探测。我们讨论了使用体积限制来探测淀粉样蛋白形成过程中的非常早期的事件。另外,可以利用尺寸在微米尺度上的通道内明确定义的流体流动性质来测量蛋白质聚集体的物理性质,例如它们的大小和电荷,以阐明定义淀粉样蛋白种类的物理和化学参数。而且,聚集反应过程中形成的分子种类的物理尺寸至少跨越三个数量级,微流体技术非常适合处理这种不同尺寸的分析物。此外,微流体装置设计的灵活性使其适用于适应性实验设置,包括研究活细胞内蛋白质的自组装。最后,我们重点介绍了有助于探测复杂生物系统的微流控实验的显着特征,并讨论了它们在探索淀粉样蛋白作为一类功能材料中的用途。包括对活细胞内蛋白质自组装的研究。最后,我们重点介绍了有助于探测复杂生物系统的微流控实验的显着特征,并讨论了它们在探索淀粉样蛋白作为一类功能材料中的用途。包括对活细胞内蛋白质自组装的研究。最后,我们重点介绍了有助于探测复杂生物系统的微流控实验的显着特征,并讨论了它们在探索淀粉样蛋白作为一类功能材料中的用途。
更新日期:2018-03-12
中文翻译:

探测淀粉样蛋白组装和行为的微流控方法
蛋白质自组装为超分子结构和机械的基础是活体系统中生物活性的基础。相比之下,错误组装,错误折叠和聚集的蛋白质结构可能具有有害的活性,此类物种是从癌症到神经退行性疾病等多种疾病的起源。特别地,由正常可溶的肽和蛋白质形成高度有序的蛋白质聚集体,淀粉样原纤维是一系列超过五十种蛋白质错误折叠障碍的常见病理学特征。由于该过程在此类疾病的病因学中的关键作用,以及对理解蛋白质折叠和错误折叠的基本原理的追求,淀粉样蛋白现象已成为现代生物医学研究的中心领域。但是,我们对淀粉样蛋白系统物理特性的了解的进步也突出了在材料科学领域中淀粉样蛋白结构的潜力。在这篇综述中,我们探讨了如何使用微流体方法来研究淀粉样蛋白组装和行为方面,这些问题在大体积溶液条件下难以探测。我们讨论了使用体积限制来探测淀粉样蛋白形成过程中的非常早期的事件。另外,可以利用尺寸在微米尺度上的通道内明确定义的流体流动性质来测量蛋白质聚集体的物理性质,例如它们的大小和电荷,以阐明定义淀粉样蛋白种类的物理和化学参数。而且,聚集反应过程中形成的分子种类的物理尺寸至少跨越三个数量级,微流体技术非常适合处理这种不同尺寸的分析物。此外,微流体装置设计的灵活性使其适用于适应性实验设置,包括研究活细胞内蛋白质的自组装。最后,我们重点介绍了有助于探测复杂生物系统的微流控实验的显着特征,并讨论了它们在探索淀粉样蛋白作为一类功能材料中的用途。包括对活细胞内蛋白质自组装的研究。最后,我们重点介绍了有助于探测复杂生物系统的微流控实验的显着特征,并讨论了它们在探索淀粉样蛋白作为一类功能材料中的用途。包括对活细胞内蛋白质自组装的研究。最后,我们重点介绍了有助于探测复杂生物系统的微流控实验的显着特征,并讨论了它们在探索淀粉样蛋白作为一类功能材料中的用途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号