Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved vaccine-induced immune responses via a ROS-triggered nanoparticle-based antigen delivery system†
Nanoscale ( IF 5.8 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1039/c8nr00355f Xiaoyu Liang 1, 2, 3, 4, 5 , Jianwei Duan 1, 2, 3, 4, 5 , Xuanling Li 1, 2, 3, 4, 5 , Xiaowei Zhu 1, 2, 3, 4, 5 , Youlu Chen 1, 2, 3, 4, 5 , Xiaoli Wang 1, 2, 3, 4, 5 , Hongfan Sun 1, 2, 3, 4, 5 , Deling Kong 1, 2, 3, 4, 5 , Chen Li 1, 2, 3, 4, 5 , Jing Yang 1, 2, 3, 4, 5
Nanoscale ( IF 5.8 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1039/c8nr00355f Xiaoyu Liang 1, 2, 3, 4, 5 , Jianwei Duan 1, 2, 3, 4, 5 , Xuanling Li 1, 2, 3, 4, 5 , Xiaowei Zhu 1, 2, 3, 4, 5 , Youlu Chen 1, 2, 3, 4, 5 , Xiaoli Wang 1, 2, 3, 4, 5 , Hongfan Sun 1, 2, 3, 4, 5 , Deling Kong 1, 2, 3, 4, 5 , Chen Li 1, 2, 3, 4, 5 , Jing Yang 1, 2, 3, 4, 5
Affiliation
|
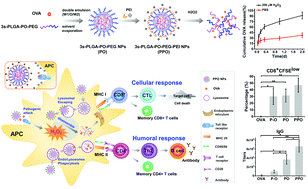
Subunit vaccines that are designed based on recombinant antigens or peptides have shown promising potential as viable substitutes for traditional vaccines due to their better safety and specificity. However, the induction of adequate in vivo immune responses with appropriate effectiveness remains a major challenge for vaccine development. More recently, the implementation of a nanoparticle-based antigen delivery system has been considered a promising approach to improve the in vivo efficacy for subunit vaccine development. Thus, we have designed and prepared a nanoparticle-based antigen delivery system composed of three-armed PLGA, which is conjugated to PEG via the peroxalate ester bond (3s-PLGA-PO-PEG) and PEI as a cationic adjuvant (PPO NPs). It is known that during a foreign pathogen attack, NADPH, an oxidase, of the host organism is activated and generates an elevated level of reactive oxygen species, hydrogen peroxide (H2O2) primarily, as a defensive mechanism. Considering the sensitivity of the peroxalate ester bond to H2O2 and the cationic property of PEI for the induction of immune responses, this 3s-PLGA-PO-PEG/PEI antigen delivery system is expected to be both ROS responsive and facilitative in antigen uptake without severe toxicity that has been reported with cationic adjuvants. Indeed, our results demonstrated excellent loading capacity and in vitro stability of the PPO NPs encapsulated with the model antigen, ovalbumin (OVA). Co-culturing of bone marrow dendritic cells with the PPO NPs also led to enhanced dendritic cell maturation, antigen uptake, enhanced lysosomal escape, antigen cross-presentation and in vitro CD8+ T cell activation. In vivo experiments using mice further revealed that the administration of the PPO nanovaccine induced robust OVA-specific antibody production, upregulation of splenic CD4+ and CD8+ T cell proportions as well as an increase in memory T cell generation. In summary, we report here a ROS-triggered nanoparticle-based antigen delivery system that could be employed to promote the in vivo efficacy of vaccine-induced immune responses.
中文翻译:

通过基于ROS触发的纳米颗粒的抗原递送系统 改善了疫苗诱导的免疫反应†
基于重组抗原或肽设计的亚单位疫苗由于其更好的安全性和特异性,已显示出有望成为传统疫苗的可行替代品的潜力。然而,诱导足够的体内免疫应答以及适当的有效性仍然是疫苗开发的主要挑战。最近,基于纳米颗粒的抗原递送系统的实施已被认为是改善亚单位疫苗开发的体内功效的有前途的方法。因此,我们设计并制备了由三臂PLGA组成的基于纳米颗粒的抗原递送系统,该三臂PLGA通过过草酸酯键(3s-PLGA-PO-PEG)和PEI作为阳离子佐剂(PPO NPs)。已知在外来病原体侵袭期间,宿主生物体的氧化酶NADPH被激活并产生升高水平的活性氧,主要是作为防御机制的过氧化氢(H 2 O 2)。考虑到过草酸酯键对H 2 O 2的敏感性和PEI诱导免疫反应的阳离子性质,该3s-PLGA-PO-PEG / PEI抗原递送系统有望在抗原中具有ROS响应性和促进性阳离子佐剂已报道其吸收而无严重毒性。确实,我们的结果证明了出色的负载能力和体外用模型抗原卵清蛋白(OVA)封装的PPO NP的稳定性。骨髓树突状细胞与PPO NP的共培养还导致树突状细胞成熟度提高,抗原摄取,溶酶体逃逸增强,抗原交叉呈递和体外CD8 + T细胞活化。使用小鼠的体内实验进一步揭示了PPO纳米疫苗的施用诱导了强劲的OVA特异性抗体产生,脾CD4 +和CD8 + T细胞比例的上调以及记忆T细胞生成的增加。总而言之,我们在这里报告一种基于ROS触发的基于纳米颗粒的抗原递送系统,该系统可用于促进体内 疫苗诱导的免疫反应的功效。
更新日期:2018-03-12
中文翻译:

通过基于ROS触发的纳米颗粒的抗原递送系统 改善了疫苗诱导的免疫反应†
基于重组抗原或肽设计的亚单位疫苗由于其更好的安全性和特异性,已显示出有望成为传统疫苗的可行替代品的潜力。然而,诱导足够的体内免疫应答以及适当的有效性仍然是疫苗开发的主要挑战。最近,基于纳米颗粒的抗原递送系统的实施已被认为是改善亚单位疫苗开发的体内功效的有前途的方法。因此,我们设计并制备了由三臂PLGA组成的基于纳米颗粒的抗原递送系统,该三臂PLGA通过过草酸酯键(3s-PLGA-PO-PEG)和PEI作为阳离子佐剂(PPO NPs)。已知在外来病原体侵袭期间,宿主生物体的氧化酶NADPH被激活并产生升高水平的活性氧,主要是作为防御机制的过氧化氢(H 2 O 2)。考虑到过草酸酯键对H 2 O 2的敏感性和PEI诱导免疫反应的阳离子性质,该3s-PLGA-PO-PEG / PEI抗原递送系统有望在抗原中具有ROS响应性和促进性阳离子佐剂已报道其吸收而无严重毒性。确实,我们的结果证明了出色的负载能力和体外用模型抗原卵清蛋白(OVA)封装的PPO NP的稳定性。骨髓树突状细胞与PPO NP的共培养还导致树突状细胞成熟度提高,抗原摄取,溶酶体逃逸增强,抗原交叉呈递和体外CD8 + T细胞活化。使用小鼠的体内实验进一步揭示了PPO纳米疫苗的施用诱导了强劲的OVA特异性抗体产生,脾CD4 +和CD8 + T细胞比例的上调以及记忆T细胞生成的增加。总而言之,我们在这里报告一种基于ROS触发的基于纳米颗粒的抗原递送系统,该系统可用于促进体内 疫苗诱导的免疫反应的功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号