当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Silico Design and in Vitro Characterization of Universal Tyrosine Kinase Peptide Substrates
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00044 Laura J. Marholz 1 , Nicholas A. Zeringo 2 , Hua Jane Lou 2 , Benjamin E. Turk 2 , Laurie L. Parker 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00044 Laura J. Marholz 1 , Nicholas A. Zeringo 2 , Hua Jane Lou 2 , Benjamin E. Turk 2 , Laurie L. Parker 1
Affiliation
|
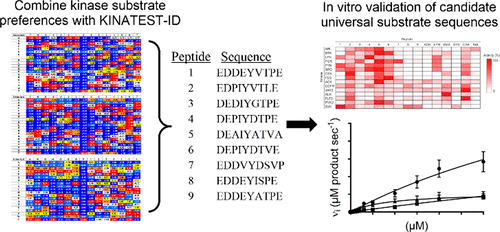
A majority of the 90 human protein tyrosine kinases (PTKs) are understudied “orphan” enzymes with few or no known substrates. Designing experiments aimed at assaying the catalytic activity of these PTKs has been a long-running problem. In the past, researchers have used polypeptides with a randomized 4:1 molar ratio of glutamic acid to tyrosine as general PTK substrates. However, these substrates are inefficient and perform poorly for many applications. In this work, we apply the KINATEST-ID pipeline for artificial kinase substrate discovery to design a set of candidate “universal” PTK peptide substrate sequences. We identified two unique peptide sequences from this set that had robust activity with a panel of 15 PTKs tested in an initial screen. Kinetic characterization with seven receptor and nonreceptor PTKs confirmed these peptides to be efficient and general PTK substrates. The broad scope of these artificial substrates demonstrates that they should be useful as tools for probing understudied PTK activity.
中文翻译:

通用酪氨酸激酶肽底物的计算机设计和体外表征
90种人类蛋白质酪氨酸激酶(PTK)中的大多数是研究不足的“孤儿”酶,几乎没有或没有已知底物。设计旨在测定这些PTK催化活性的实验是一个长期存在的问题。过去,研究人员使用谷氨酸与酪氨酸摩尔比为4:1的随机比例的多肽作为一般的PTK底物。但是,这些基板效率低下,并且在许多应用中的性能都较差。在这项工作中,我们将KINATEST-ID管道用于人工激酶底物发现,以设计一组候选“通用” PTK肽底物序列。我们从该组中鉴定出两个独特的肽序列,这些肽序列在最初的筛选中测试了15个PTK,具有强大的活性。七个受体和非受体PTK的动力学表征证实了这些肽是有效的和常规的PTK底物。这些人造底物的广泛范围表明,它们应作为探测未充分研究的PTK活性的工具而有用。
更新日期:2018-03-12
中文翻译:

通用酪氨酸激酶肽底物的计算机设计和体外表征
90种人类蛋白质酪氨酸激酶(PTK)中的大多数是研究不足的“孤儿”酶,几乎没有或没有已知底物。设计旨在测定这些PTK催化活性的实验是一个长期存在的问题。过去,研究人员使用谷氨酸与酪氨酸摩尔比为4:1的随机比例的多肽作为一般的PTK底物。但是,这些基板效率低下,并且在许多应用中的性能都较差。在这项工作中,我们将KINATEST-ID管道用于人工激酶底物发现,以设计一组候选“通用” PTK肽底物序列。我们从该组中鉴定出两个独特的肽序列,这些肽序列在最初的筛选中测试了15个PTK,具有强大的活性。七个受体和非受体PTK的动力学表征证实了这些肽是有效的和常规的PTK底物。这些人造底物的广泛范围表明,它们应作为探测未充分研究的PTK活性的工具而有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号