当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying key descriptors in surface binding: interplay of surface anchoring and intermolecular interactions for carboxylates on Au(110)†
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1039/c7sc05313d Christopher R. O'Connor 1, 2, 3, 4 , Fanny Hiebel 1, 2, 3, 4 , Wei Chen 2, 3, 4, 5, 6 , Efthimios Kaxiras 2, 3, 4, 5, 6 , Robert J. Madix 2, 3, 4, 5 , Cynthia M. Friend 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-12 00:00:00 , DOI: 10.1039/c7sc05313d Christopher R. O'Connor 1, 2, 3, 4 , Fanny Hiebel 1, 2, 3, 4 , Wei Chen 2, 3, 4, 5, 6 , Efthimios Kaxiras 2, 3, 4, 5, 6 , Robert J. Madix 2, 3, 4, 5 , Cynthia M. Friend 1, 2, 3, 4, 5
Affiliation
|
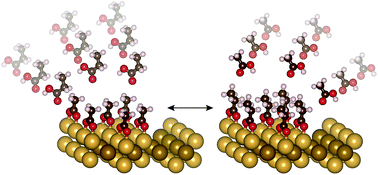
The relative stability of carboxylates on Au(110) was investigated as part of a comprehensive study of adsorbate binding on Group IB metals that can be used to predict and understand how to control reactivity in heterogeneous catalysis. The binding efficacy of carboxylates is only weakly dependent on alkyl chain length for relatively short-chain molecules, as demonstrated using quantitative temperature-programmed reaction spectroscopy. Corresponding density functional theory (DFT) calculations demonstrated that the bidentate anchoring geometry is rigid and restricts the amount of additional stabilization through adsorbate-surface van der Waals (vdW) interactions which control stability for alkoxides. A combination of scanning tunneling microscopy (STM) and low-energy electron diffraction (LEED) shows that carboxylates form dense local islands on Au(110). Complementary DFT calculations demonstrate that adsorbate–adsorbate interactions provide additional stabilization that increases as a function of alkyl chain length for C2 and C3 carboxylates. Hence, overall stability is generally a function of the anchoring group to the surface and the inter-adsorbate interaction. This study demonstrates the importance of these two important factors in describing binding of key catalytic intermediates.
中文翻译:

识别表面结合中的关键描述符:Au(110)上羧酸盐的表面锚固和分子间相互作用的相互作用†
作为对IB组金属吸附物结合的全面研究的一部分,研究了羧酸盐在Au(110)上的相对稳定性,该研究可用于预测和理解如何控制非均相催化反应。如定量温度编程反应光谱学所证明的,羧酸盐的结合功效仅相对弱地依赖于相对短链分子的烷基链长度。相应的密度泛函理论(DFT)计算表明,双齿锚固几何结构是刚性的,并且通过控制醇盐稳定性的吸附物表面范德华(vdW)相互作用限制了额外的稳定作用量。扫描隧道显微镜(STM)和低能电子衍射(LEED)的结合表明,羧酸盐在Au(110)上形成致密的局部岛。DFT的补充计算表明,吸附物与吸附物的相互作用提供了额外的稳定性,该稳定性随C的烷基链长的增加而增加2和C 3羧酸盐。因此,总体稳定性通常取决于表面上的锚固基团和吸附剂之间的相互作用。这项研究证明了这两个重要因素在描述关键催化中间体结合中的重要性。
更新日期:2018-03-12
中文翻译:

识别表面结合中的关键描述符:Au(110)上羧酸盐的表面锚固和分子间相互作用的相互作用†
作为对IB组金属吸附物结合的全面研究的一部分,研究了羧酸盐在Au(110)上的相对稳定性,该研究可用于预测和理解如何控制非均相催化反应。如定量温度编程反应光谱学所证明的,羧酸盐的结合功效仅相对弱地依赖于相对短链分子的烷基链长度。相应的密度泛函理论(DFT)计算表明,双齿锚固几何结构是刚性的,并且通过控制醇盐稳定性的吸附物表面范德华(vdW)相互作用限制了额外的稳定作用量。扫描隧道显微镜(STM)和低能电子衍射(LEED)的结合表明,羧酸盐在Au(110)上形成致密的局部岛。DFT的补充计算表明,吸附物与吸附物的相互作用提供了额外的稳定性,该稳定性随C的烷基链长的增加而增加2和C 3羧酸盐。因此,总体稳定性通常取决于表面上的锚固基团和吸附剂之间的相互作用。这项研究证明了这两个重要因素在描述关键催化中间体结合中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号