当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron Catalyzed Hydroformylation of Alkenes under Mild Conditions: Evidence of an Fe(II) Catalyzed Process
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-11 , DOI: 10.1021/jacs.8b01286 Swechchha Pandey 1 , K. Vipin Raj 2 , Dinesh R. Shinde 3 , Kumar Vanka 2 , Varchaswal Kashyap 2 , Sreekumar Kurungot 2 , C. P. Vinod 4 , Samir H. Chikkali 1, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-11 , DOI: 10.1021/jacs.8b01286 Swechchha Pandey 1 , K. Vipin Raj 2 , Dinesh R. Shinde 3 , Kumar Vanka 2 , Varchaswal Kashyap 2 , Sreekumar Kurungot 2 , C. P. Vinod 4 , Samir H. Chikkali 1, 5
Affiliation
|
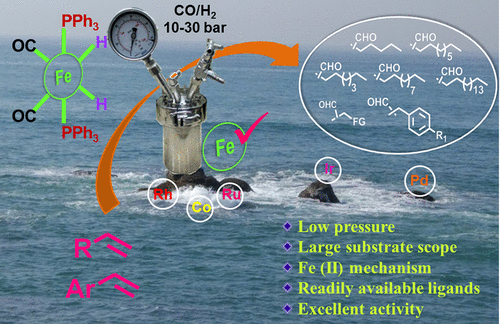
Earth abundant, first row transition metals offer a cheap and sustainable alternative to the rare and precious metals. However, utilization of first row metals in catalysis requires harsh reaction conditions, suffers from limited activity, and fails to tolerate functional groups. Reported here is a highly efficient iron catalyzed hydroformylation of alkenes under mild conditions. This protocol operates at 10-30 bar syngas pressure below 100 °C, utilizes readily available ligands, and applies to an array of olefins. Thus, the iron precursor [HFe(CO)4]-[Ph3PNPPh3]+ (1) in the presence of triphenyl phosphine catalyzes the hydroformylation of 1-hexene (S2), 1-octene (S1), 1-decene (S3), 1-dodecene (S4), 1-octadecene (S5), trimethoxy(vinyl)silane (S6), trimethyl(vinyl)silane (S7), cardanol (S8), 2,3-dihydrofuran (S9), allyl malonic acid (S10), styrene (S11), 4-methylstyrene (S12), 4- iBu-styrene (S13), 4- tBu-styrene (S14), 4-methoxy styrene (S15), 4-acetoxy styrene (S16), 4-bromo styrene (S17), 4-chloro styrene (S18), 4-vinylbenzonitrile (S19), 4-vinylbenzoic acid (S20), and allyl benzene (S21) to corresponding aldehydes in good to excellent yields. Both electron donating and electron withdrawing substituents could be tolerated and excellent conversions were obtained for S11-S20. Remarkably, the addition of 1 mol % acetic acid promotes the reaction to completion within 16-24 h. Detailed mechanistic investigations revealed in situ formation of an iron-dihydride complex [H2Fe(CO)2(PPh3)2] (A) as an active catalytic species. This finding was further supported by cyclic voltammetry investigations and intermediacy of an Fe(0)-Fe(II) species was established. Combined experimental and computational investigations support the existence of an iron-dihydride as the catalyst resting state, which then follows a Fe(II) based catalytic cycle to produce aldehyde.
中文翻译:

在温和条件下烯烃的铁催化加氢甲酰化:Fe(II) 催化过程的证据
地球上储量丰富的第一排过渡金属为稀有和贵金属提供了一种廉价且可持续的替代品。然而,在催化中使用第一行金属需要苛刻的反应条件,活性有限,并且不能耐受官能团。这里报道的是一种在温和条件下高效铁催化的烯烃加氢甲酰化反应。该协议在低于 100 °C 的 10-30 bar 合成气压力下运行,利用现成的配体,并适用于一系列烯烃。因此,铁前体 [HFe(CO)4]-[Ph3PNPPh3]+ (1) 在三苯基膦存在下催化 1-己烯 (S2)、1-辛烯 (S1)、1-癸烯 (S3) 的加氢甲酰化, 1-十二烯 (S4), 1-十八烯 (S5), 三甲氧基(乙烯基)硅烷 (S6), 三甲基(乙烯基)硅烷 (S7), 腰果酚 (S8), 2,3-二氢呋喃 (S9), 烯丙基丙二酸(S10), 苯乙烯 (S11), 4-甲基苯乙烯 (S12), 4-异丁烯-苯乙烯 (S13), 4-丁丁-苯乙烯 (S14), 4-甲氧基苯乙烯 (S15), 4-乙酰氧基苯乙烯 (S16), 4-溴苯乙烯(S17)、4-氯苯乙烯 (S18)、4-乙烯基苯甲腈 (S19)、4-乙烯基苯甲酸 (S20) 和烯丙基苯 (S21) 生成相应的醛,收率良好至极好。给电子和吸电子取代基都可以容忍,并且 S11-S20 获得了极好的转化率。值得注意的是,加入 1 mol% 的乙酸可促进反应在 16-24 小时内完成。详细的机理研究揭示了作为活性催化物质的二氢化铁络合物 [H2Fe(CO)2(PPh3)2] (A) 的原位形成。这一发现得到了循环伏安法研究的进一步支持,并建立了 Fe(0)-Fe(II) 物种的中间体。
更新日期:2018-03-11
中文翻译:

在温和条件下烯烃的铁催化加氢甲酰化:Fe(II) 催化过程的证据
地球上储量丰富的第一排过渡金属为稀有和贵金属提供了一种廉价且可持续的替代品。然而,在催化中使用第一行金属需要苛刻的反应条件,活性有限,并且不能耐受官能团。这里报道的是一种在温和条件下高效铁催化的烯烃加氢甲酰化反应。该协议在低于 100 °C 的 10-30 bar 合成气压力下运行,利用现成的配体,并适用于一系列烯烃。因此,铁前体 [HFe(CO)4]-[Ph3PNPPh3]+ (1) 在三苯基膦存在下催化 1-己烯 (S2)、1-辛烯 (S1)、1-癸烯 (S3) 的加氢甲酰化, 1-十二烯 (S4), 1-十八烯 (S5), 三甲氧基(乙烯基)硅烷 (S6), 三甲基(乙烯基)硅烷 (S7), 腰果酚 (S8), 2,3-二氢呋喃 (S9), 烯丙基丙二酸(S10), 苯乙烯 (S11), 4-甲基苯乙烯 (S12), 4-异丁烯-苯乙烯 (S13), 4-丁丁-苯乙烯 (S14), 4-甲氧基苯乙烯 (S15), 4-乙酰氧基苯乙烯 (S16), 4-溴苯乙烯(S17)、4-氯苯乙烯 (S18)、4-乙烯基苯甲腈 (S19)、4-乙烯基苯甲酸 (S20) 和烯丙基苯 (S21) 生成相应的醛,收率良好至极好。给电子和吸电子取代基都可以容忍,并且 S11-S20 获得了极好的转化率。值得注意的是,加入 1 mol% 的乙酸可促进反应在 16-24 小时内完成。详细的机理研究揭示了作为活性催化物质的二氢化铁络合物 [H2Fe(CO)2(PPh3)2] (A) 的原位形成。这一发现得到了循环伏安法研究的进一步支持,并建立了 Fe(0)-Fe(II) 物种的中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号