Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome
JAMA ( IF 63.1 ) Pub Date : 2018-04-03 , DOI: 10.1001/jama.2018.2444 Otavio Berwanger 1 , Eliana Vieira Santucci 1 , Pedro Gabriel Melo de Barros e Silva 1, 2 , Isabella de Andrade Jesuíno 1 , Lucas Petri Damiani 1 , Lilian Mazza Barbosa 2 , Renato Hideo Nakagawa Santos 1 , Ligia Nasi Laranjeira 1 , Flávia de Mattos Egydio 2 , Juliana Aparecida Borges de Oliveira 1 , Frederico Toledo Campo Dall Orto 3 , Pedro Beraldo de Andrade 4 , Igor Ribeiro de Castro Bienert 5 , Carlos Eduardo Bosso 6 , José Armando Mangione 7 , Carisi Anne Polanczyk 8 , Amanda Guerra de Moraes Rego Sousa 9 , Renato Abdala Karam Kalil 10 , Luciano de Moura Santos 11 , Andrei Carvalho Sposito 12 , Rafael Luiz Rech 13 , Antônio Carlos Sobral Sousa 14 , Felipe Baldissera 15 , Bruno Ramos Nascimento 16 , Roberto Rocha Corrêa Veiga Giraldez 17 , Alexandre Biasi Cavalcanti 1 , Sabrina Bernardez Pereira 1 , Luiz Alberto Mattos 18 , Luciana Vidal Armaganijan 2 , Hélio Penna Guimarães 1 , José Eduardo Moraes Rego Sousa 1 , John Hunter Alexander 19 , Christopher Bull Granger 19 , Renato Delascio Lopes 2, 19 ,
JAMA ( IF 63.1 ) Pub Date : 2018-04-03 , DOI: 10.1001/jama.2018.2444 Otavio Berwanger 1 , Eliana Vieira Santucci 1 , Pedro Gabriel Melo de Barros e Silva 1, 2 , Isabella de Andrade Jesuíno 1 , Lucas Petri Damiani 1 , Lilian Mazza Barbosa 2 , Renato Hideo Nakagawa Santos 1 , Ligia Nasi Laranjeira 1 , Flávia de Mattos Egydio 2 , Juliana Aparecida Borges de Oliveira 1 , Frederico Toledo Campo Dall Orto 3 , Pedro Beraldo de Andrade 4 , Igor Ribeiro de Castro Bienert 5 , Carlos Eduardo Bosso 6 , José Armando Mangione 7 , Carisi Anne Polanczyk 8 , Amanda Guerra de Moraes Rego Sousa 9 , Renato Abdala Karam Kalil 10 , Luciano de Moura Santos 11 , Andrei Carvalho Sposito 12 , Rafael Luiz Rech 13 , Antônio Carlos Sobral Sousa 14 , Felipe Baldissera 15 , Bruno Ramos Nascimento 16 , Roberto Rocha Corrêa Veiga Giraldez 17 , Alexandre Biasi Cavalcanti 1 , Sabrina Bernardez Pereira 1 , Luiz Alberto Mattos 18 , Luciana Vidal Armaganijan 2 , Hélio Penna Guimarães 1 , José Eduardo Moraes Rego Sousa 1 , John Hunter Alexander 19 , Christopher Bull Granger 19 , Renato Delascio Lopes 2, 19 ,
Affiliation
|
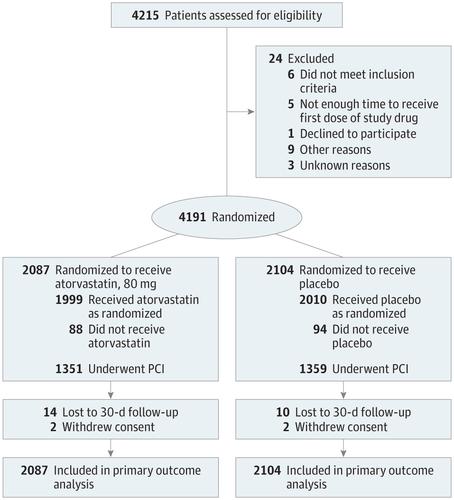
Importance The effects of loading doses of statins on clinical outcomes in patients with acute coronary syndrome (ACS) and planned invasive management remain uncertain. Objective To determine if periprocedural loading doses of atorvastatin decrease 30-day major adverse cardiovascular events (MACE) in patients with ACS and planned invasive management. Design, Setting, and Participants Multicenter, double-blind, placebo-controlled, randomized clinical trial conducted at 53 sites in Brazil among 4191 patients with ACS evaluated with coronary angiography to proceed with a percutaneous coronary intervention (PCI) if anatomically feasible. Enrollment occurred between April 18, 2012, and October 6, 2017. Final follow-up for 30-day outcomes was on November 6, 2017. Interventions Patients were randomized to receive 2 loading doses of 80 mg of atorvastatin (n = 2087) or matching placebo (n = 2104) before and 24 hours after a planned PCI. All patients received 40 mg of atorvastatin for 30 days starting 24 hours after the second dose of study medication. Main Outcomes and Measures The primary outcome was MACE, defined as a composite of all-cause mortality, myocardial infarction, stroke, and unplanned coronary revascularization through 30 days. Results Among the 4191 patients (mean age, 61.8 [SD, 11.5] years; 1085 women [25.9%]) enrolled, 4163 (99.3%) completed 30-day follow-up. A total of 2710 (64.7%) underwent PCI, 333 (8%) underwent coronary artery bypass graft surgery, and 1144 (27.3%) had exclusively medical management. At 30 days, 130 patients in the atorvastatin group (6.2%) and 149 in the placebo group (7.1%) had a MACE (absolute difference, 0.85% [95% CI, −0.70% to 2.41%]; hazard ratio, 0.88; 95% CI, 0.69-1.11; P = .27). No cases of hepatic failure were reported; 3 cases of rhabdomyolysis were reported in the placebo group (0.1%) and 0 in the atorvastatin group. Conclusions and Relevance Among patients with ACS and planned invasive management with PCI, periprocedural loading doses of atorvastatin did not reduce the rate of MACE at 30 days. These findings do not support the routine use of loading doses of atorvastatin among unselected patients with ACS and intended invasive management. Trial Registration clinicaltrials.gov Identifier: NCT01448642
中文翻译:

计划经皮冠状动脉介入治疗前阿托伐他汀负荷剂量对急性冠状动脉综合征主要心血管不良事件的影响
重要性 他汀类药物负荷剂量对急性冠状动脉综合征 (ACS) 和计划的有创治疗患者临床结局的影响仍不确定。目的 确定围手术期负荷剂量的阿托伐他汀是否能减少 ACS 和计划的侵入性治疗患者的 30 天主要不良心血管事件 (MACE)。设计、设置和参与者 在巴西 53 个地点进行的多中心、双盲、安慰剂对照、随机临床试验,对 4191 名 ACS 患者进行了冠状动脉造影评估,以便在解剖学上可行的情况下进行经皮冠状动脉介入治疗 (PCI)。入组时间为 2012 年 4 月 18 日至 2017 年 10 月 6 日。30 天结局的最终随访时间为 2017 年 11 月 6 日。干预 患者在计划 PCI 之前和之后 24 小时随机接受 2 次负荷剂量的 80 毫克阿托伐他汀(n = 2087)或匹配的安慰剂(n = 2104)。所有患者在第二剂研究药物后 24 小时开始接受 40 毫克阿托伐他汀治疗,持续 30 天。主要结局和指标 主要结局是 MACE,定义为 30 天内全因死亡率、心肌梗死、中风和非计划冠状动脉血运重建的复合。结果 在纳入的 4191 名患者(平均年龄,61.8 [SD,11.5] 岁;1085 名女性 [25.9%])中,4163 名(99.3%)完成了 30 天的随访。共有 2710 人(64.7%)接受了 PCI,333 人(8%)接受了冠状动脉搭桥手术,1144 人(27.3%)接受了完全内科治疗。在 30 天时,阿托伐他汀组 130 名患者 (6. 2%) 和安慰剂组 149 人 (7.1%) 出现 MACE(绝对差异,0.85% [95% CI,-0.70% 至 2.41%];风险比,0.88;95% CI,0.69-1.11;P = .27)。没有报告肝功能衰竭的病例;安慰剂组报告了 3 例横纹肌溶解症(0.1%),阿托伐他汀组报告了 0 例。结论和相关性 在 ACS 和计划进行 PCI 侵入性治疗的患者中,围手术期负荷剂量的阿托伐他汀并未降低 30 天的 MACE 发生率。这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 安慰剂组报告了 3 例横纹肌溶解症(0.1%),阿托伐他汀组报告了 0 例。结论和相关性 在 ACS 和计划进行 PCI 侵入性治疗的患者中,围手术期负荷剂量的阿托伐他汀并未降低 30 天的 MACE 发生率。这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 安慰剂组报告了 3 例横纹肌溶解症(0.1%),阿托伐他汀组报告了 0 例。结论和相关性 在 ACS 和计划进行 PCI 侵入性治疗的患者中,围手术期负荷剂量的阿托伐他汀并未降低 30 天的 MACE 发生率。这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642
更新日期:2018-04-03
中文翻译:

计划经皮冠状动脉介入治疗前阿托伐他汀负荷剂量对急性冠状动脉综合征主要心血管不良事件的影响
重要性 他汀类药物负荷剂量对急性冠状动脉综合征 (ACS) 和计划的有创治疗患者临床结局的影响仍不确定。目的 确定围手术期负荷剂量的阿托伐他汀是否能减少 ACS 和计划的侵入性治疗患者的 30 天主要不良心血管事件 (MACE)。设计、设置和参与者 在巴西 53 个地点进行的多中心、双盲、安慰剂对照、随机临床试验,对 4191 名 ACS 患者进行了冠状动脉造影评估,以便在解剖学上可行的情况下进行经皮冠状动脉介入治疗 (PCI)。入组时间为 2012 年 4 月 18 日至 2017 年 10 月 6 日。30 天结局的最终随访时间为 2017 年 11 月 6 日。干预 患者在计划 PCI 之前和之后 24 小时随机接受 2 次负荷剂量的 80 毫克阿托伐他汀(n = 2087)或匹配的安慰剂(n = 2104)。所有患者在第二剂研究药物后 24 小时开始接受 40 毫克阿托伐他汀治疗,持续 30 天。主要结局和指标 主要结局是 MACE,定义为 30 天内全因死亡率、心肌梗死、中风和非计划冠状动脉血运重建的复合。结果 在纳入的 4191 名患者(平均年龄,61.8 [SD,11.5] 岁;1085 名女性 [25.9%])中,4163 名(99.3%)完成了 30 天的随访。共有 2710 人(64.7%)接受了 PCI,333 人(8%)接受了冠状动脉搭桥手术,1144 人(27.3%)接受了完全内科治疗。在 30 天时,阿托伐他汀组 130 名患者 (6. 2%) 和安慰剂组 149 人 (7.1%) 出现 MACE(绝对差异,0.85% [95% CI,-0.70% 至 2.41%];风险比,0.88;95% CI,0.69-1.11;P = .27)。没有报告肝功能衰竭的病例;安慰剂组报告了 3 例横纹肌溶解症(0.1%),阿托伐他汀组报告了 0 例。结论和相关性 在 ACS 和计划进行 PCI 侵入性治疗的患者中,围手术期负荷剂量的阿托伐他汀并未降低 30 天的 MACE 发生率。这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 安慰剂组报告了 3 例横纹肌溶解症(0.1%),阿托伐他汀组报告了 0 例。结论和相关性 在 ACS 和计划进行 PCI 侵入性治疗的患者中,围手术期负荷剂量的阿托伐他汀并未降低 30 天的 MACE 发生率。这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 安慰剂组报告了 3 例横纹肌溶解症(0.1%),阿托伐他汀组报告了 0 例。结论和相关性 在 ACS 和计划进行 PCI 侵入性治疗的患者中,围手术期负荷剂量的阿托伐他汀并未降低 30 天的 MACE 发生率。这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642 这些发现不支持在未经选择的 ACS 患者中常规使用负荷剂量的阿托伐他汀并进行有创治疗。试验注册clinicaltrials.gov 标识符:NCT01448642











































 京公网安备 11010802027423号
京公网安备 11010802027423号