Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploitation of differential electronic densities for the stereoselective reduction of ketones bearing a masked amino surrogate
Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-03-09 , DOI: 10.1016/j.jcat.2018.02.014 Renta Jonathan Chew , Martin Wills
中文翻译:

利用差分电子密度立体选择性还原带有掩蔽氨基替代物的酮
更新日期:2018-03-09
Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-03-09 , DOI: 10.1016/j.jcat.2018.02.014 Renta Jonathan Chew , Martin Wills
|
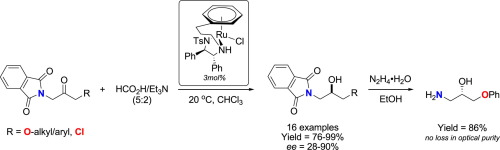
A tethered ruthenium-TsDPEN catalyst is employed for the facile catalytic asymmetric reduction of α-phthalimyl-α′-ketoethers under mild conditions. Leveraging exclusively on the contrasting electronic densities on the heteroatoms, a series of enantioenriched phthalimyl ether alcohols can be obtained in generally good stereoselectivities from this challenging class of substrate. Subsequent transformation into highly valuable chiral β-amino alcohols is demonstrated to take place without significant losses in yield and optical purity.
中文翻译:

利用差分电子密度立体选择性还原带有掩蔽氨基替代物的酮
系留钌TsDPEN催化剂用于轻便催化不对称还原的α -phthalimyl- α温和的条件下“-ketoethers。完全利用杂原子上相反的电子密度,可以从这类具有挑战性的底物上以通常良好的立体选择性获得一系列对映体富集的邻苯二甲酰亚胺醚醇。证明了随后转化为高度有价值的手性β-氨基醇的过程没有明显损失产率和光学纯度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号