Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-03-10 , DOI: 10.1016/j.bmc.2018.03.001 Shaoyi Cai , Gengzheng Zhu , Xiaohong Cen , Jingjie Bi , Jingru Zhang , Xiaoshan Tang , Kun Chen , Kui Cheng
|
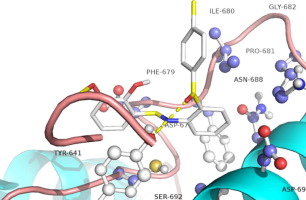
Toll-like receptor 2 (TLR2) can recognize pathogen-associated molecular patterns to defense against invading organisms and has been represents an attractive therapeutic target. Until today, none TLR2 small molecule antagonist have been developed in clinical trial. Herein, we designed and synthesized 50 N-benzylideneaniline compounds with the help of CADD. And subsequent in vitro studies leading to the optimized compound SMU-A0B13 with most potent inhibitory activity to TLR2 (IC50=18.21 ± 0.87 μM). Preliminary mechanism studies indicated that this TLR2 inhibitor can work through the NF-κB signaling pathway with high specificity and low toxicity, and can also efficiently downregulate inflammatory cytokines, such as SEAP, TNF-α and NO in HEK-Blue hTLR2, human PBMC and Raw 264.7 cell lines. Additionally, the docking situation also indicate SMU-A0B13 can well bind to the TLR2-TIR (PDB: 1FYW) active domain, which probably explains the bioactivity.
中文翻译:

N-亚苄基苯胺衍生物作为潜在的TLR2抑制剂的合成,构效关系及初步机理研究
Toll样受体2(TLR2)可以识别病原体相关的分子模式以防御入侵的生物,并且已成为有吸引力的治疗靶标。直到今天,还没有在临床试验中开发出TLR2小分子拮抗剂。本文中,我们借助CADD设计并合成了50种 N-苄叉基苯胺化合物。随后进行的体外研究产生了对TLR2具有最强抑制活性的优化化合物SMU-A0B13(IC 50= 18.21±0.87μM)。初步的机制研究表明,该TLR2抑制剂可通过NF-κB信号通路发挥高特异性和低毒性的作用,并且还可以有效下调HEK-blue hTLR2,人PBMC和SHE中的炎性细胞因子,例如SEAP,TNF-α和NO。原始的264.7细胞系。此外,对接情况还表明SMU-A0B13可以很好地与TLR2-TIR(PDB:1FYW)活性域结合,这可能解释了其生物活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号