Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2018-03-10 , DOI: 10.1016/j.apcatb.2018.03.032 Yuanyuan Wang , Qian Zhu , Yan Wei , Yanjun Gong , Chuncheng Chen , Wenjing Song , Jincai Zhao
|
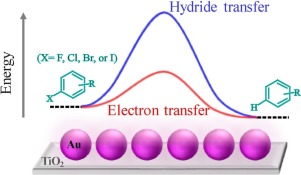
Selective hydrodehalogenation of a variety of aromatic halides (e.g., halogen subsitute phenones and hexafluorobenzene) using isopropanol as the hydrogen source was successfully achieved over supported Au nanoparticles. Distinct reaction pathways were established based on kinetic analysis and intermediate identification. Surface hydrides generated by isopropanol oxidation mediate the hydrodehalogenation by nucleophilic attack without the involvement of carbon centered radical. On the other hand, engeretic electrons on Au nanoparticles populated by visible light irradiation trigger the dissciation of carbon halogen bond (CX) by injection to the substrates’ lowest unoccupied molecular orbitals (LUMO). The super-linear dependency on light intensity, shape of the action spectrum and formation of aryl radical revealed that electron transfer dominates the hydrodehalogneation process, which features a significantly lower activation barrier than transfer of surface hydride. The observed activity is correlated to energy levels of the substrate electron accepting states.
中文翻译:

负载金的催化加氢脱卤:电子转移与氢化物转移
在负载的金纳米粒子上成功实现了使用异丙醇作为氢源的多种芳香族卤化物(例如,卤素取代的苯甲酮和六氟苯)的选择性加氢脱卤。基于动力学分析和中间鉴定建立了不同的反应途径。异丙醇氧化生成的表面氢化物可通过亲核攻击介导加氢脱卤作用,而不会涉及以碳为中心的自由基。另一方面,可见光辐照在金纳米粒子上的充能电子触发了碳卤键(CX)通过注入底物的最低未占据分子轨道(LUMO)。对光强度,作用谱的形状和芳基自由基的形成的超线性依赖性表明,电子转移在加氢脱卤过程中起着主导作用,该过程的活化势垒明显低于表面氢化物的转移。观察到的活性与底物电子接受态的能级相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号