当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Oxoglutarate regulates binding of hydroxylated hypoxia-inducible factor to prolyl hydroxylase domain 2†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1039/c8cc00387d Martine I Abboud 1 , Tom E McAllister 1 , Ivanhoe K H Leung 2 , Rasheduzzaman Chowdhury 1 , Christian Jorgensen 3 , Carmen Domene 4 , Jasmin Mecinović 5 , Kerstin Lippl 1 , Rebecca L Hancock 1 , Richard J Hopkinson 6 , Akane Kawamura 1 , Timothy D W Claridge 1 , Christopher J Schofield 1
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1039/c8cc00387d Martine I Abboud 1 , Tom E McAllister 1 , Ivanhoe K H Leung 2 , Rasheduzzaman Chowdhury 1 , Christian Jorgensen 3 , Carmen Domene 4 , Jasmin Mecinović 5 , Kerstin Lippl 1 , Rebecca L Hancock 1 , Richard J Hopkinson 6 , Akane Kawamura 1 , Timothy D W Claridge 1 , Christopher J Schofield 1
Affiliation
|
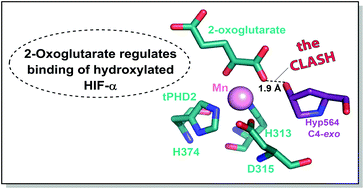
Prolyl hydroxylation of hypoxia inducible factor (HIF)-α, as catalysed by the Fe(II)/2-oxoglutarate (2OG)-dependent prolyl hydroxylase domain (PHD) enzymes, has a hypoxia sensing role in animals. We report that binding of prolyl-hydroxylated HIF-α to PHD2 is ∼50 fold hindered by prior 2OG binding; thus, when 2OG is limiting, HIF-α degradation might be inhibited by PHD binding.
中文翻译:

2-氧戊二酸调节羟基化缺氧诱导因子与脯氨酰羟化酶结构域的结合 2†
由 Fe( II )/2-氧戊二酸 (2OG) 依赖性脯氨酰羟化酶结构域 (PHD) 酶催化的缺氧诱导因子 (HIF)-α 的脯氨酰羟基化在动物中具有缺氧感应作用。我们报告脯氨酰羟基化 HIF-α 与 PHD2 的结合受到先前 2OG 结合的阻碍约 50 倍。因此,当 2OG 受到限制时,HIF-α 降解可能会被 PHD 结合抑制。
更新日期:2018-03-09
中文翻译:

2-氧戊二酸调节羟基化缺氧诱导因子与脯氨酰羟化酶结构域的结合 2†
由 Fe( II )/2-氧戊二酸 (2OG) 依赖性脯氨酰羟化酶结构域 (PHD) 酶催化的缺氧诱导因子 (HIF)-α 的脯氨酰羟基化在动物中具有缺氧感应作用。我们报告脯氨酰羟基化 HIF-α 与 PHD2 的结合受到先前 2OG 结合的阻碍约 50 倍。因此,当 2OG 受到限制时,HIF-α 降解可能会被 PHD 结合抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号