Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
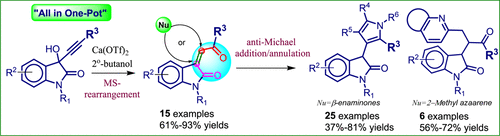
Highly Regioselective Synthesis of Oxindolyl-Pyrroles and Quinolines via a One-Pot, Sequential Meyer-Schuster Rearrangement, Anti-Michael Addition/C(sp3)-H Functionalization, and Azacyclization.
ACS Omega ( IF 3.7 ) Pub Date : 2018-03-09 , DOI: 10.1021/acsomega.8b00147 Srinivasarao Yaragorla 1 , Ravikrishna Dada 1 , P Rajesh 1 , Manju Sharma 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-03-09 , DOI: 10.1021/acsomega.8b00147 Srinivasarao Yaragorla 1 , Ravikrishna Dada 1 , P Rajesh 1 , Manju Sharma 1
Affiliation
|
A one-pot, sequential Meyer-Schuster (MS) rearrangement of oxindole-derived propargyl alcohols to the corresponding α,β-unsaturated enones and their anti-Michael addition, followed by intramolecular azacyclization is described in a highly regioselective manner using Ca(OTf)2 as the promoter. Further, we described the one-pot MS rearrangement, followed by C(sp3)-H functionalization of 2-methyl azaarenes at α-carbon of these doubly activated alkenes. Control experiments and computational calculations were performed to propose the reaction mechanism.
中文翻译:

通过一锅,顺序迈耶-舒斯特重排,反迈克尔加成/ C(sp3)-H功能化和氮杂环化的高区域选择性合成的Oxindolyl吡咯和喹啉。
使用Ca(OTf)在区域上高度选择性地描述了羟吲哚衍生的炔丙醇与相应的α,β-不饱和烯酮的一锅,连续Meyer-Schuster(MS)重排及其抗Michael加成反应,然后进行了分子内氮杂环化反应。 )2作为发起人。此外,我们描述了一锅MS重排,然后在这些双活化烯烃的α-碳上对2-甲基氮杂芳烃进行C(sp3)-H官能化。进行了控制实验和计算计算以提出反应机理。
更新日期:2018-03-09
中文翻译:

通过一锅,顺序迈耶-舒斯特重排,反迈克尔加成/ C(sp3)-H功能化和氮杂环化的高区域选择性合成的Oxindolyl吡咯和喹啉。
使用Ca(OTf)在区域上高度选择性地描述了羟吲哚衍生的炔丙醇与相应的α,β-不饱和烯酮的一锅,连续Meyer-Schuster(MS)重排及其抗Michael加成反应,然后进行了分子内氮杂环化反应。 )2作为发起人。此外,我们描述了一锅MS重排,然后在这些双活化烯烃的α-碳上对2-甲基氮杂芳烃进行C(sp3)-H官能化。进行了控制实验和计算计算以提出反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号