Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-03-10 , DOI: 10.1016/j.actbio.2018.03.006 Jonas Reinholz , Christopher Diesler , Susanne Schöttler , Maria Kokkinopoulou , Sandra Ritz , Katharina Landfester , Volker Mailänder
|
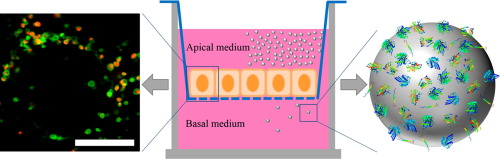
The transport of nanocarriers through barriers like the gut in a living organism involves the transcytosis of these nanocarriers through the cell layer dividing two compartments. Understanding how this process works is not only essential to further developing strategies for a more effective nanocarrier transport system but also for providing fundamental insights into the barrier function as a means of protection against micro- and nanoplastics in the food chain. We therefore set out to investigate the different uptake mechanisms, intracellular trafficking and the routes for exocytosis for small polystyrene nanoparticles (PS-NPs ca. 100 nm) as mimicking nanocarriers in a Caco-2 cell model for gut-blood transition. We used label-free, quantitative mass spectrometry (MS) for determining the proteome that adhered to transversed nanoparticles. From this rich proteomics dataset, as well as previous studies, we generated stable-transfected Caco-2 cell lines carrying the green fluorescent protein (GFP) coupled to proteins of interest for uptake, early, late and exocytotic endosomes. We detected the spatial and temporal overlap of such marked endosomes with the nanocarrier signal in confocal laser scanning and super-resolution microscopy. There was a clear distinction in the time course of nanoparticle trafficking between groups of proteins for endocytosis, intracellular storage and putatively transcytosis and we identified several key transcytotic markers like Rab3 and Copine1. Moreover, we postulate the necessity of a certain protein composition on endosomes for successful transcytosis of nanocarriers. Finally, we define the two-sided impasse of the lysosome as a dead end for nano-plastic and the limit of nanocarriers in the 100 nm range.
Significance Transcytosis
Here we focus on mechanisms of transcytosis and how we can follow these with methods not used before. First, we use mass spectrometry of transcytosed nanoparticles to pick proteins of the transcytosis machinery describing key proteins involved. We can detect the complex mixtures of proteins. As this is a dynamic process involving whole families of proteins interacting with each other and as this is an orchestrated process we coined the term protein machineries for this active interplay.
By genetically modifying the proteins attaching GFP we are able to follow the transcytosis pathway We evaluate the process in a quantitative manner over time. This reveals that the most obvious obstacle to transcytosis is a routing of the nanocarriers to the lysosomes.
中文翻译:

定义纳米载体胞吐和转胞吞途径的蛋白质机制
纳米载体通过诸如肠道在生物体内的屏障的转运涉及通过分隔两个部分的细胞层对这些纳米载体的胞吞作用。理解该过程的工作方式,不仅对于进一步开发更有效的纳米载体运输系统的策略至关重要,而且对于提供屏障功能作为对食物链中的微米和纳米塑料的防护手段的基础见解也至关重要。因此,我们着手研究不同的摄取机制,细胞内运输和小聚苯乙烯纳米颗粒(PS-NP约100 nm)作为模仿肠道血液过渡的Caco-2细胞模型中的纳米载体的胞吐途径。我们使用无标记的定量质谱(MS)来确定粘附在横向纳米颗粒上的蛋白质组。从这个丰富的蛋白质组学数据集以及以前的研究中,我们生成了稳定转染的Caco-2细胞系,该细胞系带有绿色荧光蛋白(GFP)和感兴趣的蛋白质,可用于摄取,早期,晚期和胞吐内体。我们在共聚焦激光扫描和超分辨率显微镜中检测到这种标记的内体与纳米载体信号的空间和时间重叠。在用于内吞,胞内存储和推定的胞吞作用的蛋白质组之间,纳米颗粒运输的时间进程存在明显区别,我们鉴定了一些关键的胞吞标记物,如Rab3和Copine1。此外,我们假设内体上必须具有某种蛋白质成分才能成功转座纳米载体。最后,
有意义的胞吞作用
在这里,我们集中于转胞吞作用的机制,以及如何使用以前未使用的方法来遵循这些机制。首先,我们使用质谱分析了转胞吞的纳米颗粒,以选择描述相关关键蛋白的转胞吞机制的蛋白质。我们可以检测出复杂的蛋白质混合物。由于这是一个涉及整个蛋白质家族相互作用的动态过程,因此,由于我们是精心策划的过程,因此我们创造了术语“蛋白质机制”来表达这种活跃的相互作用。
通过遗传修饰连接GFP的蛋白质,我们能够遵循转胞吞途径。随着时间的流逝,我们以定量的方式评估了这一过程。这揭示了转胞吞作用的最明显障碍是纳米载体向溶酶体的路由。











































 京公网安备 11010802027423号
京公网安备 11010802027423号