当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Rational Design of Highly Controlled Suzuki-Miyaura Catalyst-Transfer Polycondensation for Precision Synthesis of Polythiophenes and their Block Copolymers: Marriage of Palladacycle Precatalysts with MIDA-boronates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-09 , DOI: 10.1021/jacs.7b13701 Kyeong-Bae Seo 1 , In-Hwan Lee 2 , Jaeho Lee 1 , Inho Choi 1 , Tae-Lim Choi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-09 , DOI: 10.1021/jacs.7b13701 Kyeong-Bae Seo 1 , In-Hwan Lee 2 , Jaeho Lee 1 , Inho Choi 1 , Tae-Lim Choi 1
Affiliation
|
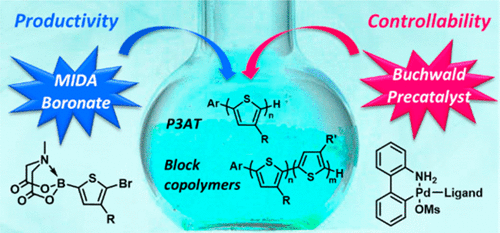
Herein, we report a highly efficient Suzuki-Miyaura catalyst-transfer polycondensation (SCTP) of 3-alkylthiophenes using bench-stable but highly active Buchwald dialkylbiarylphospine Pd G3 precatalysts and N-methylimidodiacetic (MIDA)-boronate monomers. Initially, the feasibility of the catalyst-transfer process was examined by screening various dialkylbiarylphospine-Pd(0) species. After optimizing a small molecule model reaction, we identified both RuPhos and SPhos Pd G3 precatalysts as excellent catalyst systems for this purpose. On the basis of these model studies, SCTP was tested using either RuPhos or SPhos Pd G3 precatalyst, and 5-bromo-4- n-hexylthien-2-yl-pinacol-boronate. Poly(3-hexylthiophene) (P3HT) was produced with controlled molecular weight and narrow dispersity for a low degree of polymerization (DP) only, while attempts to synthesize P3HT having a higher DP with good control were unsuccessful. To improve the control, slowly hydrolyzed 5-bromo-4- n-hexylthien-2-yl-MIDA-boronate was introduced as a new monomer. As a result, P3HT and P3EHT (up to 17.6 kg/mol) were prepared with excellent control, narrow dispersity, and excellent yield (>90%). Detailed mechanistic investigation using 31P NMR and MALDI-TOF spectroscopy revealed that both fast initiation using Buchwald precatalysts and the suppression of protodeboronation due to the protected MIDA-boronate were crucial to achieve successful living polymerization of P3HT. In addition, a block copolymer of P3HT- b-P3EHT was prepared via SCTP by sequential addition of each MIDA-boronate monomer. Furthermore, the same block copolymer was synthesized by one-shot copolymerization for the first time by using fast propagating pinacol-boronate and slow propagating MIDA-boronate.
中文翻译:

用于精密合成聚噻吩及其嵌段共聚物的高度可控的 Suzuki-Miyaura 催化剂转移缩聚反应的合理设计:钯环预催化剂与 MIDA-硼酸酯的结合
在此,我们报告了一种高效的 Suzuki-Miyaura 催化剂转移缩聚反应 (SCTP),使用台架稳定但活性高的 Buchwald 二烷基联芳基膦 Pd G3 预催化剂和 N-甲基亚胺二乙酸 (MIDA)-硼酸酯单体。最初,通过筛选各种二烷基二芳基膦-Pd(0) 物种来检查催化剂转移过程的可行性。在优化小分子模型反应后,我们将 RuPhos 和 SPhos Pd G3 预催化剂鉴定为用于此目的的出色催化剂体系。在这些模型研究的基础上,使用 RuPhos 或 SPhos Pd G3 预催化剂和 5-bromo-4-n-hexylthien-2-yl-pinacol-boronate 测试 SCTP。聚(3-己基噻吩)(P3HT)仅在低聚合度(DP)时具有受控的分子量和窄分散性,而试图合成具有良好控制的更高 DP 的 P3HT 的尝试没有成功。为了改善控制,引入缓慢水解的 5-bromo-4-n-hexylthien-2-yl-MIDA-boronate 作为新单体。因此,制备的 P3HT 和 P3EHT(高达 17.6 kg/mol)具有出色的控制性、窄分散性和出色的产率(>90%)。使用 31P NMR 和 MALDI-TOF 光谱进行的详细机理研究表明,使用 Buchwald 预催化剂的快速引发和由于受保护的 MIDA 硼酸盐对原脱硼的抑制对于实现 P3HT 的成功活性聚合至关重要。此外,P3HT-b-P3EHT 的嵌段共聚物是通过 SCTP 通过顺序添加每种 MIDA-硼酸酯单体来制备的。此外,
更新日期:2018-03-09
中文翻译:

用于精密合成聚噻吩及其嵌段共聚物的高度可控的 Suzuki-Miyaura 催化剂转移缩聚反应的合理设计:钯环预催化剂与 MIDA-硼酸酯的结合
在此,我们报告了一种高效的 Suzuki-Miyaura 催化剂转移缩聚反应 (SCTP),使用台架稳定但活性高的 Buchwald 二烷基联芳基膦 Pd G3 预催化剂和 N-甲基亚胺二乙酸 (MIDA)-硼酸酯单体。最初,通过筛选各种二烷基二芳基膦-Pd(0) 物种来检查催化剂转移过程的可行性。在优化小分子模型反应后,我们将 RuPhos 和 SPhos Pd G3 预催化剂鉴定为用于此目的的出色催化剂体系。在这些模型研究的基础上,使用 RuPhos 或 SPhos Pd G3 预催化剂和 5-bromo-4-n-hexylthien-2-yl-pinacol-boronate 测试 SCTP。聚(3-己基噻吩)(P3HT)仅在低聚合度(DP)时具有受控的分子量和窄分散性,而试图合成具有良好控制的更高 DP 的 P3HT 的尝试没有成功。为了改善控制,引入缓慢水解的 5-bromo-4-n-hexylthien-2-yl-MIDA-boronate 作为新单体。因此,制备的 P3HT 和 P3EHT(高达 17.6 kg/mol)具有出色的控制性、窄分散性和出色的产率(>90%)。使用 31P NMR 和 MALDI-TOF 光谱进行的详细机理研究表明,使用 Buchwald 预催化剂的快速引发和由于受保护的 MIDA 硼酸盐对原脱硼的抑制对于实现 P3HT 的成功活性聚合至关重要。此外,P3HT-b-P3EHT 的嵌段共聚物是通过 SCTP 通过顺序添加每种 MIDA-硼酸酯单体来制备的。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号