当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trends in the Catalytic Activity of Hydrogen Evolution during CO2 Electroreduction on Transition Metals
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acscatal.7b03807 Etosha R. Cave 1, 2 , Chuan Shi 1, 2 , Kendra P. Kuhl 1, 2 , Toru Hatsukade 1, 2 , David N. Abram 1, 2 , Christopher Hahn 1, 2 , Karen Chan 1, 2 , Thomas F. Jaramillo 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acscatal.7b03807 Etosha R. Cave 1, 2 , Chuan Shi 1, 2 , Kendra P. Kuhl 1, 2 , Toru Hatsukade 1, 2 , David N. Abram 1, 2 , Christopher Hahn 1, 2 , Karen Chan 1, 2 , Thomas F. Jaramillo 1, 2
Affiliation
|
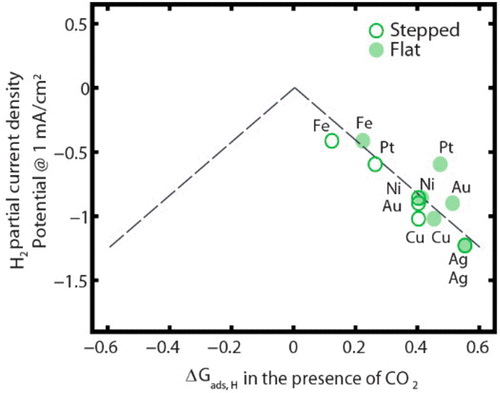
During CO2 electroreduction (CO2R), the hydrogen evolution reaction (HER) is a competing reaction. We present a combined experimental and theoretical investigation of the HER activity of transition metals under CO2R conditions. Experimental HER polarization curves were measured for six polycrystalline metal surfaces (Au, Ag, Cu, Ni, Pt, and Fe) in the presence of CO2 gas. We found that the HER activity of the transition metals is significantly shifted, relative to the CO2-free case. Density functional theory (DFT) calculations suggest that this shift arises from adsorbate–adsorbate interactions between *CO and *H on intermediate and strong binding metals, which weakens the *H binding energy. Using a simple model for the effect of *CO on the *H binding energy, we construct an activity volcano for HER in the presence of CO2 gas that is consistent with experimental trends. The significant changes in HER activity in the presence of CO2 gas is an important consideration in catalyst design and could help develop catalysts that are more selective for CO2R than the HER.
中文翻译:

过渡金属在CO 2电还原过程中析氢的催化活性趋势
在CO 2电还原(CO 2 R)过程中,析氢反应(HER)是竞争反应。我们提出了在CO 2 R条件下过渡金属的HER活性的组合实验和理论研究。在存在CO 2气体的情况下,针对六个多晶金属表面(Au,Ag,Cu,Ni,Pt和Fe)测量了HER极化实验曲线。我们发现,过渡金属的HER活性相对于CO 2发生了显着变化-无案。密度泛函理论(DFT)的计算表明,这种转变是由于* CO和* H在中间和强结合金属上的吸附物-吸附物相互作用而产生的,这削弱了* H的结合能。使用简单的* CO对* H结合能影响的模型,我们在存在CO 2气体的情况下构建了与实验趋势一致的HER活火山。在存在CO 2气体的情况下,HER活性的显着变化是催化剂设计中的重要考虑因素,并且可以帮助开发对HER 2的选择性比HER高的催化剂。
更新日期:2018-03-09
中文翻译:

过渡金属在CO 2电还原过程中析氢的催化活性趋势
在CO 2电还原(CO 2 R)过程中,析氢反应(HER)是竞争反应。我们提出了在CO 2 R条件下过渡金属的HER活性的组合实验和理论研究。在存在CO 2气体的情况下,针对六个多晶金属表面(Au,Ag,Cu,Ni,Pt和Fe)测量了HER极化实验曲线。我们发现,过渡金属的HER活性相对于CO 2发生了显着变化-无案。密度泛函理论(DFT)的计算表明,这种转变是由于* CO和* H在中间和强结合金属上的吸附物-吸附物相互作用而产生的,这削弱了* H的结合能。使用简单的* CO对* H结合能影响的模型,我们在存在CO 2气体的情况下构建了与实验趋势一致的HER活火山。在存在CO 2气体的情况下,HER活性的显着变化是催化剂设计中的重要考虑因素,并且可以帮助开发对HER 2的选择性比HER高的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号