当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Accuracy of Amorphous Solubility Advantage Calculation by Comparison with Experimental Solubility Measurement in Buffer and Biorelevant Media
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00125 Wei Zhang 1 , Abbe Haser 2 , Hao Helen Hou 1 , Karthik Nagapudi 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00125 Wei Zhang 1 , Abbe Haser 2 , Hao Helen Hou 1 , Karthik Nagapudi 1
Affiliation
|
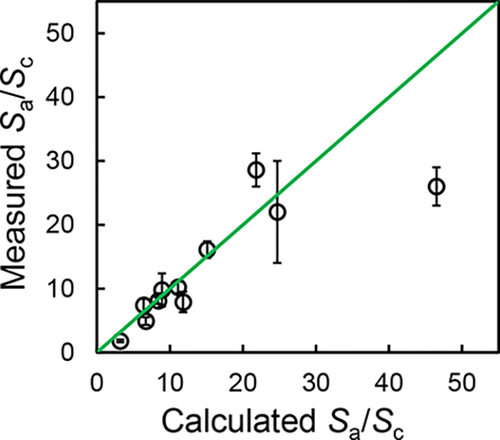
The accuracy of amorphous solubility advantage calculation was evaluated by experimental solubility measurement. Ten structurally diverse compounds were studied to test the generlity of the theoretical calculation. Three reported methods of calculating Gibbs free energy difference between amorphous and crystalline solids were evaluated. Experimental solubility advantage was measured by direct dissolution of amorphous solid in buffer. When necessary, hydroxypropyl methylcellulose acetate succinate (HPMCAS) was predissolved in buffer to inhibit desupersaturation. By direct dissolution, the effect of different preparation methods on amorphous solubility was also studied. Finally, solubility measurement was performed in fasted state simulated intestinal fluid (FaSSIF) to assess the effect of bile salt on the concentration-based amorphous solubility advantage. The results showed that the assumption of constant heat capacity differences between crystal and supercooled liquid or amorphous solid is sufficient for accurate theoretical calculation, which is attributed to the fact that the heat capacity of crystal is nearly parallel to that of supercooled liquid or amorphous solid. Different preparation methods do not have significant impact on amorphous solubility advantage. Experimental measurement agrees with the theoretical calculation within a factor of 0.7 to 1.8. The concentration-based amorphous solubility advantage in FaSSIF agrees well with theoretical calculation. This work demonstrates that theoretical calculation of amorphous solubility advantage is robust and can be applied in early drug development for assessing the utility of the amorphous phase.
中文翻译:

通过与缓冲液和生物相关介质中的实验溶解度测量结果进行比较,评估非晶态溶解度优势计算的准确性
通过实验溶解度测量评估非晶态溶解度优势计算的准确性。研究了十种结构多样的化合物,以检验理论计算的广泛性。评价了三种报告的计算非晶态和结晶态固体之间吉布斯自由能差的方法。通过将无定形固体直接溶解在缓冲液中来测量实验的溶解度优势。必要时,将羟丙基甲基纤维素乙酸琥珀酸酯(HPMCAS)预溶解在缓冲液中以抑制过饱和。通过直接溶解,还研究了不同制备方法对无定形溶解度的影响。最后,在禁食状态的模拟肠液(FaSSIF)中进行溶解度测量,以评估胆汁盐对基于浓度的无定形溶解度优势的影响。结果表明,晶体与过冷液体或非晶态固体之间的热容量差恒定的假设足以进行精确的理论计算,这是由于晶体的热容量几乎与过冷液体或非晶态固体的热容量平行。不同的制备方法对无定形溶解度优势没有显着影响。实验测量值与理论计算结果相符,范围为0.7到1.8。FaSSIF中基于浓度的非晶态溶解度优势与理论计算非常吻合。
更新日期:2018-03-09
中文翻译:

通过与缓冲液和生物相关介质中的实验溶解度测量结果进行比较,评估非晶态溶解度优势计算的准确性
通过实验溶解度测量评估非晶态溶解度优势计算的准确性。研究了十种结构多样的化合物,以检验理论计算的广泛性。评价了三种报告的计算非晶态和结晶态固体之间吉布斯自由能差的方法。通过将无定形固体直接溶解在缓冲液中来测量实验的溶解度优势。必要时,将羟丙基甲基纤维素乙酸琥珀酸酯(HPMCAS)预溶解在缓冲液中以抑制过饱和。通过直接溶解,还研究了不同制备方法对无定形溶解度的影响。最后,在禁食状态的模拟肠液(FaSSIF)中进行溶解度测量,以评估胆汁盐对基于浓度的无定形溶解度优势的影响。结果表明,晶体与过冷液体或非晶态固体之间的热容量差恒定的假设足以进行精确的理论计算,这是由于晶体的热容量几乎与过冷液体或非晶态固体的热容量平行。不同的制备方法对无定形溶解度优势没有显着影响。实验测量值与理论计算结果相符,范围为0.7到1.8。FaSSIF中基于浓度的非晶态溶解度优势与理论计算非常吻合。









































 京公网安备 11010802027423号
京公网安备 11010802027423号