当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomaterial Scaffolds as Pre‐metastatic Niche Mimics Systemically Alter the Primary Tumor and Tumor Microenvironment
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-03-09 , DOI: 10.1002/adhm.201700903 Brian A Aguado 1 , Rachel M Hartfield 2 , Grace G Bushnell 2 , Joseph T Decker 2 , Samira M Azarin 3 , Dhaval Nanavati 4 , Matthew J Schipma 5 , Shreyas S Rao 6 , Robert S Oakes 2 , Yining Zhang 2 , Jacqueline S Jeruss 7 , Lonnie D Shea 2, 8
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-03-09 , DOI: 10.1002/adhm.201700903 Brian A Aguado 1 , Rachel M Hartfield 2 , Grace G Bushnell 2 , Joseph T Decker 2 , Samira M Azarin 3 , Dhaval Nanavati 4 , Matthew J Schipma 5 , Shreyas S Rao 6 , Robert S Oakes 2 , Yining Zhang 2 , Jacqueline S Jeruss 7 , Lonnie D Shea 2, 8
Affiliation
|
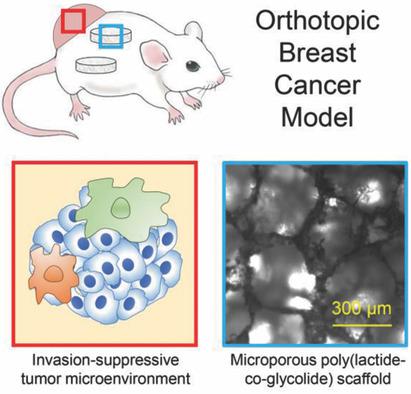
Primary tumor (PT) immune cells and pre‐metastatic niche (PMN) sites are critical to metastasis. Recently, synthetic biomaterial scaffolds used as PMN mimics are shown to capture both immune and metastatic tumor cells. Herein, studies are performed to investigate whether the scaffold‐mediated redirection of immune and tumor cells would alter the primary tumor microenvironment (TME). Transcriptomic analysis of PT cells from scaffold‐implanted and mock‐surgery mice identifies differentially regulated pathways relevant to invasion and metastasis progression. Transcriptomic differences are hypothesized to result from scaffold‐mediated modulations of immune cell trafficking and phenotype in the TME. Culturing tumor cells with conditioned media generated from PT immune cells of scaffold‐implanted mice decrease invasion in vitro more than two‐fold relative to mock surgery controls and reduce activity of invasion‐promoting transcription factors. Secretomic characterization of the conditioned media delineates interactions between immune cells in the TME and tumor cells, showing an increase in the pan‐metastasis inhibitor decorin and a concomitant decrease in invasion‐promoting chemokine (C‐C motif) ligand 2 (CCL2) in scaffold‐implanted mice. Flow cytometric and transcriptomic profiling of PT immune cells identify phenotypically distinct tumor‐associated macrophages (TAMs) in scaffold‐implanted mice, which may contribute to an invasion‐suppressive TME. Taken together, this study demonstrates biomaterial scaffolds systemically influence metastatic progression through manipulation of the TME.
中文翻译:

生物材料支架作为转移前生态位模拟系统地改变原发肿瘤和肿瘤微环境
原发肿瘤(PT)免疫细胞和转移前微环境(PMN)位点对于转移至关重要。最近,用作 PMN 模拟物的合成生物材料支架被证明可以捕获免疫和转移性肿瘤细胞。在此,进行研究以调查支架介导的免疫细胞和肿瘤细胞的重定向是否会改变原发肿瘤微环境(TME)。对支架植入小鼠和模拟手术小鼠的 PT 细胞进行转录组分析,确定了与侵袭和转移进展相关的差异调节途径。假设转录组差异是由支架介导的 TME 中免疫细胞运输和表型调节引起的。用支架植入小鼠的 PT 免疫细胞产生的条件培养基培养肿瘤细胞,与模拟手术对照相比,体外侵袭减少了两倍以上,并降低了侵袭促进转录因子的活性。条件培养基的分泌组学特征描绘了 TME 中的免疫细胞与肿瘤细胞之间的相互作用,显示支架中全转移抑制剂核心蛋白聚糖的增加以及伴随的侵袭促进趋化因子(C-C 基序)配体 2 (CCL2) 的减少‐植入小鼠。 PT 免疫细胞的流式细胞术和转录组学分析可识别支架植入小鼠中表型不同的肿瘤相关巨噬细胞 (TAM),这可能有助于抑制侵袭性 TME。总而言之,这项研究表明生物材料支架通过 TME 的操作系统地影响转移进展。
更新日期:2018-03-09
中文翻译:

生物材料支架作为转移前生态位模拟系统地改变原发肿瘤和肿瘤微环境
原发肿瘤(PT)免疫细胞和转移前微环境(PMN)位点对于转移至关重要。最近,用作 PMN 模拟物的合成生物材料支架被证明可以捕获免疫和转移性肿瘤细胞。在此,进行研究以调查支架介导的免疫细胞和肿瘤细胞的重定向是否会改变原发肿瘤微环境(TME)。对支架植入小鼠和模拟手术小鼠的 PT 细胞进行转录组分析,确定了与侵袭和转移进展相关的差异调节途径。假设转录组差异是由支架介导的 TME 中免疫细胞运输和表型调节引起的。用支架植入小鼠的 PT 免疫细胞产生的条件培养基培养肿瘤细胞,与模拟手术对照相比,体外侵袭减少了两倍以上,并降低了侵袭促进转录因子的活性。条件培养基的分泌组学特征描绘了 TME 中的免疫细胞与肿瘤细胞之间的相互作用,显示支架中全转移抑制剂核心蛋白聚糖的增加以及伴随的侵袭促进趋化因子(C-C 基序)配体 2 (CCL2) 的减少‐植入小鼠。 PT 免疫细胞的流式细胞术和转录组学分析可识别支架植入小鼠中表型不同的肿瘤相关巨噬细胞 (TAM),这可能有助于抑制侵袭性 TME。总而言之,这项研究表明生物材料支架通过 TME 的操作系统地影响转移进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号