当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Platinum‐Catalysed Ring‐Opening Isomerisation of Piperidine Cyclopropanes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-03-09 , DOI: 10.1002/ajoc.201700706 Viktor Barát 1 , Sivarajan Kasinathan 1 , Roderick W. Bates 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-03-09 , DOI: 10.1002/ajoc.201700706 Viktor Barát 1 , Sivarajan Kasinathan 1 , Roderick W. Bates 1
Affiliation
|
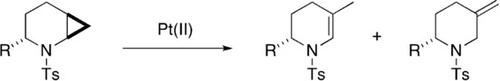
A range of cyclopropyl‐fused N‐tosyl piperidines have been synthesised and shown to undergo ring‐opening isomerisation on treatment with platinum(II) catalysts. The products can have either an endo‐cyclic or exo‐cyclic double bond. The selectivity is influenced by reaction temperature, solvent and, most significantly, the catalyst. A mechanism involving C−C bond activation and β‐hydride elimination is proposed. When β‐hydride elimination is blocked, a stereospecific platinum‐driven Wagner–Meerwein shift is observed.
中文翻译:

铂催化哌啶环丙烷的开环异构化
已合成了一系列环丙基稠合的N-甲苯磺酰基哌啶,并显示在用铂(II)催化剂处理后会发生开环异构化。该产品可以有一个内切-环状或外-环双键。选择性受反应温度,溶剂和最重要的是催化剂的影响。提出了涉及CC键活化和β-氢化物消除的机理。当阻止β-氢化物消除时,会观察到立体定向的铂驱动的Wagner-Meerwein位移。
更新日期:2018-03-09
中文翻译:

铂催化哌啶环丙烷的开环异构化
已合成了一系列环丙基稠合的N-甲苯磺酰基哌啶,并显示在用铂(II)催化剂处理后会发生开环异构化。该产品可以有一个内切-环状或外-环双键。选择性受反应温度,溶剂和最重要的是催化剂的影响。提出了涉及CC键活化和β-氢化物消除的机理。当阻止β-氢化物消除时,会观察到立体定向的铂驱动的Wagner-Meerwein位移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号