当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of a chiral macromolecular catalyst in hollow silica nanoreactors for efficient and recyclable asymmetric catalysis†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1039/c7cy02610b Lingyan Jing 1, 2, 3, 4 , Xiaoming Zhang 1, 2, 3, 4 , Ruqun Guan 1, 2, 3, 4 , Hengquan Yang 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1039/c7cy02610b Lingyan Jing 1, 2, 3, 4 , Xiaoming Zhang 1, 2, 3, 4 , Ruqun Guan 1, 2, 3, 4 , Hengquan Yang 1, 2, 3, 4
Affiliation
|
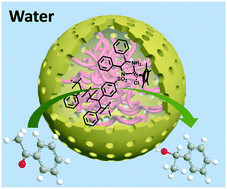
The entrapment method is a fascinating pathway to convert homogeneous into heterogeneous catalysts. However, the types of entrapped catalysts and available supports are still limited because of the strict requirements of this method. Here, we reported a novel strategy for achieving efficient entrapment of a chiral homogeneous catalyst by forming macromolecular polymers in the cavity of hollow silica nanoreactors. Specifically, through in situ polymerization of the chiral ligand (1R,2R)-N1-(4-vinylbenzenesulfonyl)-1,2-diphenylethane-1,2-diamine (VBS-DPEN) with styrene followed by coordination of the metal precursor [Cp*RhCl2]2 (Cp* = pentamethylcyclopentadiene), a chiral solid catalyst with a confined macromolecular polymer was obtained. In the aqueous asymmetric transfer hydrogenation (ATH) of ketones, such a catalyst exhibited much better activity (TOF 991 vs. 724 h−1) and similar enantioselectivity (94% ee) compared with its homogeneous counterpart. This excellent activity can be attributed to the combination of the hollow mesoporous structure, unique hydrophobic@hydrophilic surface properties and semi-free state of the macromolecular catalyst. Moreover, the solid catalyst exhibits good recyclability, which is much better than the pure macromolecular polymer catalyst. Our studies not only provide an excellent heterogeneous asymmetric catalyst but also demonstrate that the pathway for confining macromolecules could be used as an efficient scaffold for the synthesis of entrapped catalysts.
中文翻译:

在空心二氧化硅纳米反应器中构建手性高分子催化剂,以进行有效且可回收的不对称催化†
捕集方法是将均相转化为非均相催化剂的一种引人入胜的途径。但是,由于该方法的严格要求,所夹带的催化剂和可用载体的类型仍然受到限制。在这里,我们报告了一种通过在空心二氧化硅纳米反应器的空腔中形成高分子聚合物来有效捕获手性均相催化剂的新策略。具体而言,通过手性配体(1 R,2 R)-N 1-(4-乙烯基苯磺酰基)-1,2-二苯乙烷-1,2-二胺(VBS-DPEN)与苯乙烯的原位聚合,然后与金属前体[Cp * RhCl 2 ] 2(Cp * =五甲基环戊二烯),得到具有受限的高分子聚合物的手性固体催化剂。在酮的水不对称转移加氢(ATH)中,这种催化剂表现出更好的活性(TOF 991 vs. 724 h -1)和相对应的对映选择性(94%ee)。这种优异的活性可以归因于大分子催化剂的中空介孔结构,独特的疏水@亲水表面特性和半自由状态的组合。而且,固体催化剂显示出良好的可循环性,这比纯的大分子聚合物催化剂好得多。我们的研究不仅提供了出色的非均相不对称催化剂,而且证明了限制大分子的途径可以用作合成被困催化剂的有效支架。
更新日期:2018-03-09
中文翻译:

在空心二氧化硅纳米反应器中构建手性高分子催化剂,以进行有效且可回收的不对称催化†
捕集方法是将均相转化为非均相催化剂的一种引人入胜的途径。但是,由于该方法的严格要求,所夹带的催化剂和可用载体的类型仍然受到限制。在这里,我们报告了一种通过在空心二氧化硅纳米反应器的空腔中形成高分子聚合物来有效捕获手性均相催化剂的新策略。具体而言,通过手性配体(1 R,2 R)-N 1-(4-乙烯基苯磺酰基)-1,2-二苯乙烷-1,2-二胺(VBS-DPEN)与苯乙烯的原位聚合,然后与金属前体[Cp * RhCl 2 ] 2(Cp * =五甲基环戊二烯),得到具有受限的高分子聚合物的手性固体催化剂。在酮的水不对称转移加氢(ATH)中,这种催化剂表现出更好的活性(TOF 991 vs. 724 h -1)和相对应的对映选择性(94%ee)。这种优异的活性可以归因于大分子催化剂的中空介孔结构,独特的疏水@亲水表面特性和半自由状态的组合。而且,固体催化剂显示出良好的可循环性,这比纯的大分子聚合物催化剂好得多。我们的研究不仅提供了出色的非均相不对称催化剂,而且证明了限制大分子的途径可以用作合成被困催化剂的有效支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号