当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Teaching Electrochemistry in the General Chemistry Laboratory through Corrosion Exercises
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.jchemed.7b00416 Richard W. Sanders 1 , Gregory L. Crettol 1 , Joseph D. Brown 1 , Patrick T. Plummer 1 , Tara M. Schendorf 1 , Alex Oliphant 1 , Susan B. Swithenbank 2 , Robert F. Ferrante 3 , Joshua P. Gray 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.jchemed.7b00416 Richard W. Sanders 1 , Gregory L. Crettol 1 , Joseph D. Brown 1 , Patrick T. Plummer 1 , Tara M. Schendorf 1 , Alex Oliphant 1 , Susan B. Swithenbank 2 , Robert F. Ferrante 3 , Joshua P. Gray 1
Affiliation
|
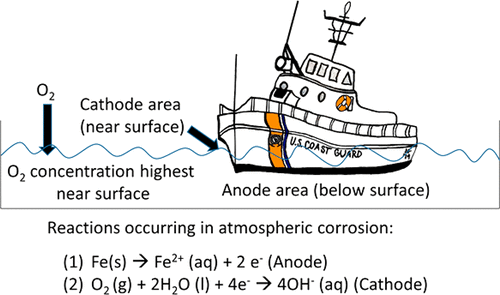
Electrochemistry is primarily taught in first-year undergraduate courses through batteries; this lab focuses instead on corrosion to apply electrochemical concepts of electrolytes, standard reduction potentials, galvanic cells, and other chemistry concepts including Le Chatelier’s Principle and Henry’s Law. Students investigate galvanic corrosion under neutral and acidic conditions quantitatively by measuring voltage, amperage, and mass loss and qualitatively using phenolphthalein. They also investigate protection against corrosion through the use of sacrificial anodes and impressed current cathodic protection.
中文翻译:

通过腐蚀练习在普通化学实验室中教授电化学
电化学主要在大学一年级课程中通过电池进行讲授。该实验室重点研究腐蚀,以应用电解质的电化学概念,标准还原电势,原电池以及其他化学概念,包括勒沙特利尔原理和亨利定律。学生可以通过测量电压,安培数和质量损失并定性地使用酚酞来定量研究中性和酸性条件下的电偶腐蚀。他们还研究了通过使用牺牲阳极和施加外加电流阴极保护来防止腐蚀的方法。
更新日期:2018-03-09
中文翻译:

通过腐蚀练习在普通化学实验室中教授电化学
电化学主要在大学一年级课程中通过电池进行讲授。该实验室重点研究腐蚀,以应用电解质的电化学概念,标准还原电势,原电池以及其他化学概念,包括勒沙特利尔原理和亨利定律。学生可以通过测量电压,安培数和质量损失并定性地使用酚酞来定量研究中性和酸性条件下的电偶腐蚀。他们还研究了通过使用牺牲阳极和施加外加电流阴极保护来防止腐蚀的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号