当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlled Transformations of Aryl Halides in a Flow System: Selective Synthesis of Aryl Azides and Aniline Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-04-06 , DOI: 10.1002/adsc.201701539 Ádám Georgiádes 1 , Sándor B. Ötvös 1, 2 , Ferenc Fülöp 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-04-06 , DOI: 10.1002/adsc.201701539 Ádám Georgiádes 1 , Sándor B. Ötvös 1, 2 , Ferenc Fülöp 1, 2
Affiliation
|
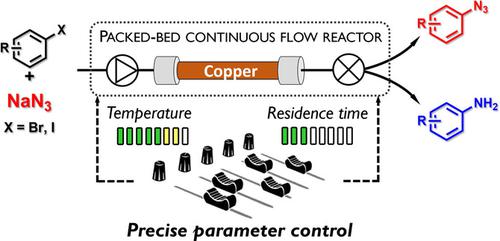
Copper‐mediated nitrogenation of aryl halides with sodium azide can result in either aryl azides or aniline derivatives. The selectivity of the transformation is highly dependent on reaction time and temperature, which led to contradictory literature results with respect to product selectivity and the conditions applied. The advantages of a strictly controlled flow reactor environment were exploited in order to facilitate selective haloarene transformations. Reaction conditions were carefully investigated to understand their role on product selectivity. Aryl azides and aryl amines were successfully prepared from the same starting materials using the same auxiliaries by means of precise control over residence time and reaction temperature, thereby ensuring time‐, cost‐ and atom‐efficient syntheses.
中文翻译:

流动体系中芳基卤化物的受控转化:芳基叠氮化物和苯胺衍生物的选择性合成
铜介导的叠氮化钠对芳基卤化物的氮化可导致芳基叠氮化物或苯胺衍生物。转化的选择性高度依赖于反应时间和温度,这导致关于产物选择性和所用条件的文献结果相互矛盾。为了促进选择性卤代芳烃的转化,利用了严格控制的流动反应器环境的优点。仔细研究了反应条件,以了解它们对产物选择性的作用。通过精确控制停留时间和反应温度,从相同的原料使用相同的助剂成功制备了芳基叠氮化物和芳基胺,从而确保了时间,成本和原子效率的合成。
更新日期:2018-04-06
中文翻译:

流动体系中芳基卤化物的受控转化:芳基叠氮化物和苯胺衍生物的选择性合成
铜介导的叠氮化钠对芳基卤化物的氮化可导致芳基叠氮化物或苯胺衍生物。转化的选择性高度依赖于反应时间和温度,这导致关于产物选择性和所用条件的文献结果相互矛盾。为了促进选择性卤代芳烃的转化,利用了严格控制的流动反应器环境的优点。仔细研究了反应条件,以了解它们对产物选择性的作用。通过精确控制停留时间和反应温度,从相同的原料使用相同的助剂成功制备了芳基叠氮化物和芳基胺,从而确保了时间,成本和原子效率的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号