当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifunctional trastuzumab–chlorin e6 conjugate for the treatment of HER2-positive human breast cancer†
Biomaterials Science ( IF 5.8 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1039/c7bm01084b Kyoung Sub Kim 1, 2, 3, 4, 5 , Jiyoung Kim 1, 2, 3, 4, 5 , Da Hye Kim 1, 2, 3, 4, 5 , Hee Sook Hwang 1, 2, 3, 4, 5 , Kun Na 1, 2, 3, 4, 5
Biomaterials Science ( IF 5.8 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1039/c7bm01084b Kyoung Sub Kim 1, 2, 3, 4, 5 , Jiyoung Kim 1, 2, 3, 4, 5 , Da Hye Kim 1, 2, 3, 4, 5 , Hee Sook Hwang 1, 2, 3, 4, 5 , Kun Na 1, 2, 3, 4, 5
Affiliation
|
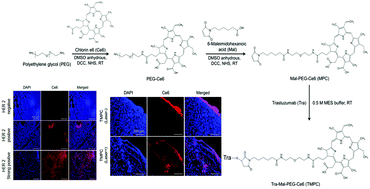
Effective penetration and targeted delivery of anticancer drugs into tumor tissues are limiting factors for achieving enhanced therapeutic efficacy. In order to overcome the disadvantages of antibody therapy (limited penetration efficacy into tumor tissues) and photodynamic therapy (low targeting efficiency) on the treatment of HER2-positive human breast cancer simultaneously, an antibody and photosensitizer combined Trastuzumab–chlorin e6 conjugate (TMPC) was synthesized. TMPC exhibits high singlet oxygen generation under laser irradiation. In vitro data show that TMPC has specific HER2 selective interactions, and ROS generation ability upon laser irradiation induces significant cell death in HER2-positive breast cancer cells. The enhanced tissue penetration ability and tissue access of TMPC resulting from local tissue destruction by ROS generated from Ce6 is also demonstrated in breast cancer tissue blocks. The enhanced ability of TMPC is supported by in vivo fluorescence images of SK-BR-3 (HER2-positive cancer cells) tumor-bearing mice. The in vivo test demonstrates approximately 6-fold enhanced accumulation of TMPC in xenograft tumors with a longer retention time compared to that of the PEG–Ce6 conjugate at 24 h. Thus, trastuzumab and photosensitizer conjugation brings synergistic effects for HER2 selective targeting, where TMPC enhanced tumor tissue penetration improves tumor tissue access and responsiveness of trastuzumab in HER2 overexpressing breast cancer.
中文翻译:

多功能曲妥珠单抗-二氢卟酚e6缀合物用于治疗HER2阳性人类乳腺癌†
抗癌药对肿瘤组织的有效渗透和靶向递送是实现增强的治疗功效的限制因素。为了克服同时治疗HER2阳性人类乳腺癌的抗体疗法(限制进入肿瘤组织的穿透力)和光动力疗法(靶向效率低)的缺点,抗体和光敏剂联合曲妥珠单抗-二氢卟酚e6缀合物(TMPC)是合成的。TMPC在激光辐照下表现出高的单线态氧生成。体外数据显示TMPC具有特定的HER2选择性相互作用,并且激光照射后ROS的产生能力会在HER2阳性乳腺癌细胞中诱导明显的细胞死亡。在乳腺癌组织块中还证实了由于Ce6产生的ROS引起的局部组织破坏而导致的TMPC组织渗透能力增强和组织通路。携带SK-BR-3(HER2阳性癌细胞)的荷瘤小鼠的体内荧光图像支持TMPC的增强功能。在体内试验证明,与24小时PEG-Ce6缀合物相比,TMPC在异种移植肿瘤中的积累增加了约6倍,保留时间更长。因此,曲妥珠单抗和光敏剂的缀合为HER2选择性靶向带来协同作用,其中TMPC增强的肿瘤组织渗透性改善了曲妥珠单抗在HER2过表达的乳腺癌中的肿瘤组织通路和反应性。
更新日期:2018-03-09
中文翻译:

多功能曲妥珠单抗-二氢卟酚e6缀合物用于治疗HER2阳性人类乳腺癌†
抗癌药对肿瘤组织的有效渗透和靶向递送是实现增强的治疗功效的限制因素。为了克服同时治疗HER2阳性人类乳腺癌的抗体疗法(限制进入肿瘤组织的穿透力)和光动力疗法(靶向效率低)的缺点,抗体和光敏剂联合曲妥珠单抗-二氢卟酚e6缀合物(TMPC)是合成的。TMPC在激光辐照下表现出高的单线态氧生成。体外数据显示TMPC具有特定的HER2选择性相互作用,并且激光照射后ROS的产生能力会在HER2阳性乳腺癌细胞中诱导明显的细胞死亡。在乳腺癌组织块中还证实了由于Ce6产生的ROS引起的局部组织破坏而导致的TMPC组织渗透能力增强和组织通路。携带SK-BR-3(HER2阳性癌细胞)的荷瘤小鼠的体内荧光图像支持TMPC的增强功能。在体内试验证明,与24小时PEG-Ce6缀合物相比,TMPC在异种移植肿瘤中的积累增加了约6倍,保留时间更长。因此,曲妥珠单抗和光敏剂的缀合为HER2选择性靶向带来协同作用,其中TMPC增强的肿瘤组织渗透性改善了曲妥珠单抗在HER2过表达的乳腺癌中的肿瘤组织通路和反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号