当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neutron Crystallography Detects Differences in Protein Dynamics: Structure of the PKG II Cyclic Nucleotide Binding Domain in Complex with an Activator
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.biochem.8b00010 Oksana Gerlits 1 , James C. Campbell 2 , Matthew P. Blakeley 3 , Choel Kim 2, 4 , Andrey Kovalevsky 5
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.biochem.8b00010 Oksana Gerlits 1 , James C. Campbell 2 , Matthew P. Blakeley 3 , Choel Kim 2, 4 , Andrey Kovalevsky 5
Affiliation
|
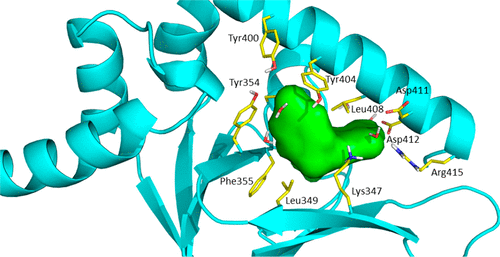
As one of the main receptors of a second messenger, cGMP, cGMP-dependent protein kinase (PKG) isoforms I and II regulate distinct physiological processes. The design of isoform-specific activators is thus of great biomedical importance and requires detailed structural information about PKG isoforms bound with activators, including accurate positions of hydrogen atoms and a description of the hydrogen bonding and water architecture. Here, we determined a 2.2 Å room-temperature joint X-ray/neutron (XN) structure of the human PKG II carboxyl cyclic nucleotide binding (CNB-B) domain bound with a potent PKG II activator, 8-pCPT-cGMP. The XN structure directly visualizes intermolecular interactions and reveals changes in hydrogen bonding patterns upon comparison to the X-ray structure determined at cryo-temperatures. Comparative analysis of the backbone hydrogen/deuterium exchange patterns in PKG II:8-pCPT-cGMP and previously reported PKG Iβ:cGMP XN structures suggests that the ability of these agonists to activate PKG is related to how effectively they quench dynamics of the cyclic nucleotide binding pocket and the surrounding regions.
中文翻译:

中子晶体学检测蛋白质动力学的差异:与激活剂复杂的PKG II环核苷酸结合域的结构。
作为第二信使的主要受体之一,cGMP依赖于cGMP的蛋白激酶(PKG)异构体I和II调节不同的生理过程。因此,异构体特异性活化剂的设计具有重要的生物医学意义,并且需要有关与活化剂结合的PKG异构体的详细结构信息,包括氢原子的准确位置以及氢键和水结构的描述。在这里,我们确定了与有效PKG II激活剂8-pCPT-cGMP结合的人PKG II羧基环状核苷酸结合(CNB-B)结构域的2.2室温室温X射线/中子(XN)结构。与在低温下测定的X射线结构相比,XN结构直接可视化了分子间的相互作用并揭示了氢键模式的变化。
更新日期:2018-03-08
中文翻译:

中子晶体学检测蛋白质动力学的差异:与激活剂复杂的PKG II环核苷酸结合域的结构。
作为第二信使的主要受体之一,cGMP依赖于cGMP的蛋白激酶(PKG)异构体I和II调节不同的生理过程。因此,异构体特异性活化剂的设计具有重要的生物医学意义,并且需要有关与活化剂结合的PKG异构体的详细结构信息,包括氢原子的准确位置以及氢键和水结构的描述。在这里,我们确定了与有效PKG II激活剂8-pCPT-cGMP结合的人PKG II羧基环状核苷酸结合(CNB-B)结构域的2.2室温室温X射线/中子(XN)结构。与在低温下测定的X射线结构相比,XN结构直接可视化了分子间的相互作用并揭示了氢键模式的变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号