当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P450-Catalyzed Regio- and Diastereoselective Steroid Hydroxylation: Efficient Directed Evolution Enabled by Mutability Landscaping
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acscatal.8b00389 Carlos G. Acevedo-Rocha 1, 2 , Charles G. Gamble 3 , Richard Lonsdale 1, 2, 4 , Aitao Li 1, 2, 5 , Nathalie Nett 2 , Sabrina Hoebenreich 2 , Julia B. Lingnau 1 , Cornelia Wirtz 1 , Christophe Fares 1 , Heike Hinrichs 1 , Alfred Deege 1 , Adrian J. Mulholland 4 , Yuval Nov 6 , David Leys 3 , Kirsty J. McLean 3 , Andrew W. Munro 3 , Manfred T. Reetz 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acscatal.8b00389 Carlos G. Acevedo-Rocha 1, 2 , Charles G. Gamble 3 , Richard Lonsdale 1, 2, 4 , Aitao Li 1, 2, 5 , Nathalie Nett 2 , Sabrina Hoebenreich 2 , Julia B. Lingnau 1 , Cornelia Wirtz 1 , Christophe Fares 1 , Heike Hinrichs 1 , Alfred Deege 1 , Adrian J. Mulholland 4 , Yuval Nov 6 , David Leys 3 , Kirsty J. McLean 3 , Andrew W. Munro 3 , Manfred T. Reetz 1, 2
Affiliation
|
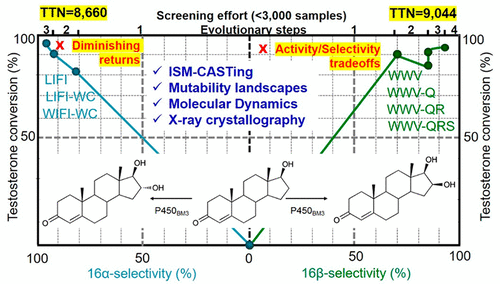
Cytochrome P450 monooxygenases play a crucial role in the biosynthesis of many natural products and in the human metabolism of numerous pharmaceuticals. This has inspired synthetic organic and medicinal chemists to exploit them as catalysts in regio- and stereoselective CH-activating oxidation of structurally simple and complex organic compounds such as steroids. However, levels of regio- and stereoselectivity as well as activity are not routinely high enough for real applications. Protein engineering using rational design or directed evolution has helped in many respects, but simultaneous engineering of multiple catalytic traits such as activity, regioselectivity, and stereoselectivity, while overcoming trade-offs and diminishing returns, remains a challenge. Here we show that the exploitation of information derived from mutability landscapes and molecular dynamics simulations for rationally designing iterative saturation mutagenesis constitutes a viable directed evolution strategy. This combined approach is illustrated by the evolution of P450BM3 mutants which enable nearly perfect regio- and diastereoselective hydroxylation of five different steroids specifically at the C16-position with unusually high activity, while avoiding activity–selectivity trade-offs as well as keeping the screening effort relatively low. The C16 alcohols are of practical interest as components of biologically active glucocorticoids.
中文翻译:

P450催化的区域和非对映体选择性甾体羟化反应:可变性美化使高效定向进化成为可能
细胞色素P450单加氧酶在许多天然产物的生物合成和许多药物的人类代谢中起着至关重要的作用。这激发了合成有机和药物化学家将其用作结构简单和复杂有机化合物(如类固醇)的区域和立体选择性CH活化氧化的催化剂。然而,对于实际应用而言,区域选择性和立体选择性以及活性水平通常不够高。使用合理设计或定向进化的蛋白质工程在很多方面都有所帮助,但是同时克服多种催化特性(例如活性,区域选择性和立体选择性)的工程设计,同时克服了取舍和收益递减的情况,仍然是一个挑战。在这里,我们表明,利用变异性景观信息和分子动力学模拟信息来合理设计迭代饱和诱变,是一种可行的定向进化策略。P450的演进说明了这种组合方法BM3突变体可实现五个不同类固醇的几乎完美的区域和非对映选择性羟基化,特别是在C16位置,具有异常高的活性,同时避免了活性-选择性之间的权衡并保持相对较低的筛选工作。作为生物活性糖皮质激素的组分,C16醇具有实际意义。
更新日期:2018-03-08
中文翻译:

P450催化的区域和非对映体选择性甾体羟化反应:可变性美化使高效定向进化成为可能
细胞色素P450单加氧酶在许多天然产物的生物合成和许多药物的人类代谢中起着至关重要的作用。这激发了合成有机和药物化学家将其用作结构简单和复杂有机化合物(如类固醇)的区域和立体选择性CH活化氧化的催化剂。然而,对于实际应用而言,区域选择性和立体选择性以及活性水平通常不够高。使用合理设计或定向进化的蛋白质工程在很多方面都有所帮助,但是同时克服多种催化特性(例如活性,区域选择性和立体选择性)的工程设计,同时克服了取舍和收益递减的情况,仍然是一个挑战。在这里,我们表明,利用变异性景观信息和分子动力学模拟信息来合理设计迭代饱和诱变,是一种可行的定向进化策略。P450的演进说明了这种组合方法BM3突变体可实现五个不同类固醇的几乎完美的区域和非对映选择性羟基化,特别是在C16位置,具有异常高的活性,同时避免了活性-选择性之间的权衡并保持相对较低的筛选工作。作为生物活性糖皮质激素的组分,C16醇具有实际意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号